
|
시장보고서
상품코드
1458501
<2024> 바이폴라전지 기술개발 현황 및 향후 전망<2024> Current Status and Future Outlook of Bipolar Battery Technology Development |
||||||
집전체의 양면에 같은 전극으로 구성되는 모노폴라 전극으로 이루어진 싱글 셀로 대표되는 2차전지는 모든 전극이 같은 전해질에 담가져 있고, 각 전극은 외부 연결선을 사용하여 병렬로 연결되어 많은 비활성 물질이 배터리 시스템에 통합되어 결과적으로 부피에너지밀도는 약 40%, 중량에너지밀도는 약 20%의 손실이 추정됩니다.
바이폴라 배터리는 전기 커넥터 및 기타 액세서리를 사용하지 않아 셀 구성과 모양이 심플하며, 배터리 부피는 전체 단위셀의 적층 두께와 단위셀의 기판 면적의 곱에 가깝고, 배터리 무게는 모든 구성 요소의 질량 합계에 가깝습니다. 바이폴라 배터리의 용량은 단일 단위셀의 용량과 동일하지만, 바이폴라 배터리의 출력전압은 직렬로 연결된 단위셀의 수와 각 셀의 전압의 곱에 의해 결정됩니다.
바이폴라 전극을 사용하는 배터리는 부피/중량 에너지밀도도 크게 증가합니다. 또한 애플리케이션 중심 설계를 기반으로 배터리 모양을 쉽게 조정할 수 있어 대상 장치의 배터리 저장 공간 활용도가 극대화됩니다. 즉, 배터리 부피가 감소되며, BMS를 최소화할 수 있어서, 셀 외장재 사용 최소화로 에너지밀도 향상과 단가 절약을 동시에 추구할 수 있습니다. 이는 곧 한정된 전기차 배터리 탑재 공간에 더 많은 배터리를 탑재할 수 있게 돼 주행거리 증가로 이어질 수 있을 것으로 기대할 수 있습니다. 따라서 바이폴라 전극의 이러한 장점은 모바일 전자 제품 및 전기 자동차에 사용되는 2차 전지 설계에 매우 매력적입니다.
바이폴라 전극의 또 다른 장점은 전자 흐름이 기판에 수직적으로 이루어지며, 기판의 단면적이 크면 전류 밀도와 분포가 크게 향상됩니다. 따라서 바이폴라 전극을 사용하면 작동 속도가 빠른 2차 전지는 안전 문제없이 작동할 수 있습니다.
바이폴라 전극을 적용한 Furukawa전기의 납축전지를 시작으로 최근에 TOYOTA에서 바이폴라 Ni-MH전지를 상용화하여 Aqua HEV에 적용하였으며, ‘23년 6월 발표에서는 Volume급 EV에 바이폴라 LFP를 ‘26-’27년에 생산하고, 미래버전의 EV에는 바이폴라 Ni계 LiB를 ‘27-’28년에 생산하여 성능버전의 LiB와 비교하여 주행거리와 코스트를 향상시킨다는 로드맵을 발표한 바 있습니다.
최근 출시된 TOYOTA 크라운 크로스오버와 렉서스(Lexus RX)에는 기존의 Ni-MH를 개량한 바이폴라 Ni-MH가 장착되었는데, 고급 모델, 고연비 중심 모델에는 LIB를 적용해 왔던 것과 반대되는 행보로 점차 라인업 전반에 Ni-MH배터리를 확대 탑재하겠다는 의미로 해석됩니다. 본 보고서에서는 이제 막 적용이 시작된 바이폴라전극 개발의 역사와 지금까지의 연구개발현황 등을 수록하였고, 각 개발의 내용을 좀 더 자세하게 다루어 전체적인 현황을 쉽게 파악할 수 있도록 구성하였습니다.
본 보고서의 Strong Point는 다음과 같습니다.
- ① 바이폴라 전지를 둘러싼 최근의 기술 동향을 상세히 수록
- ② 바이폴라 전지 개발사들의 개발이력과 개발 현황을 상세히 수록
- ③ TOYOTA 자동차의 바이폴라 전지 개발 현황을 집중 수록
- ④ 바이폴라 전지의 주요 특허 분석
목 차
1. 이차전지용 바이폴라(Bipolar)전극
- 1.1. 배터리구조 최적화 필요
- 1.2. 바이폴라 전극
- 1.3. 바이폴라 전극 개발
- 1.3.1. 바이폴라 전극 개발 역사
- 1.3.2. 무게, 크기, 비용 감소
- 1.3.3. 에너지밀도/출력밀도 향상
- 1.3.4. 바이폴라 전극 요구사항 및 단점
- 1.4. 바이폴라 전극의 응용
- 1.4.1. 바이폴라 납축전지(LAB)
- 1.4.2. 바이폴라 납축전지 개선
- 1.4.2.1. 표면 개질
- 1.4.2.2. 부식 방지
- 1.4.3. 바이폴라 납축전지 상용화
- 1.5. 바이폴라 알카리 배터리
- 1.5.1. 바이폴라 Ni-MH
- 1.5.2. 바이폴라 Al 및 Zn 배터리
- 1.6. 바이폴라 리튬이온전지(LIB)
- 1.7. 바이폴라 post-LiB(Li-S, Na-ion)
- 1.8. 과제 및 전망
- 1.8.1. 기판 재료
- 1.8.2. 전극 재료
- 1.8.3. 전해질 재료
- 1.8.4. 엔지니어링 기술
- 1.8.5. 바이폴라 전극 전망
- 1.8.6. 실용화에의 허들
- 1.8.7. 기타 바이폴라 전지
- 1.8.8. 바이폴라 전고체전지
2. 바이폴라 전고체전지: 설계, 제작 및 전기화학
- 2.1. 개요
- 2.1.1. 바이폴라 전고체전지 장점
- 2.1.2. 바이폴라 배터리의 기술적 과제
- 2.1.3. 바이폴라 재료의 요구사항
- 2.2. 바이폴라 플레이트
- 2.3. 바이폴라 전고체전지 제작 및 전기화학적 특성
- 2.3.1. Free standing lamination 바이폴라 전고체전지
- 2.3.2. 프린팅 바이폴라 전고체전지
- 2.4. 결과 및 향후 전망
3. 바이폴라 전고체전지: 에너지밀도 설계 Toolkit
- 3.1. 개요
- 3.2. 결과 및 토론
- 3.2.1. SolidPAC demo
- 3.2.2. 바이폴라 적층 및 기존 적층 비교
- 3.2.3. 민감도 분석
- 3.2.4. 실험 데이터 분석
4. 바이폴라 전고체전지: 준고체 전해질 기반
- 4.1. 준고체 Li-Glyme complex
- 4.2. 바이폴라 전고체 리튬전지 평가
- 4.3. 바이폴라 전고체 리튬전지 SEM 분석
- 4.4. 결론
5. 바이폴라 전고체전지: 황화물계 전해질 기반
- 5.1. 개요
- 5.2. 결과 및 토론
- 5.2.1. 양극층 제조 및 특성
- 5.2.2. 음극층 제조 및 특성
- 5.2.3. 모노셀 제조 및 특성
- 5.2.4. 바이폴라 적층 전고체전지 특성
- 5.2.5. 바이폴라 전고체전지 에너지밀도 비교
- 5.3. 결론
6. 바이폴라 전고체전지: Multistage printing 제조 기반
- 6.1. 개요
- 6.2. Introduction
- 6.3. 실험
- 6.3.1. SWCNT 코팅 전극활물질 제조
- 6.3.2. Printed 바이폴라 LIB 제작
- 6.4. 결과 및 토론
- 6.4.1. 고체 겔복합전해질(GCE)
- 6.4.2. Printed 전극의 제작 및 특성
- 6.4.3. GCE 및 전극 페이스트 컨트롤
- 6.4.4. 기계적 유연성 및 열적 안정성
- 6.5. 결론
7. 바이폴라 전고체전지: FeOx-LFBO 음극 적용
- 7.1. 개요
- 7.2. Introduction
- 7.3. 실험 결과
- 7.3.1. FeOx-LFBO 합성 및 특성
- 7.3.2. FeOx-LFBO 음극의 전기화학적 성능
- 7.3.3. 메커니즘 분석
- 7.3.4. Cu-free LIB의 전기화학적 성능
- 7.4. 결론
8. 바이폴라 LFP/LTO 전지: Micro/Mild Hybrid용 LIB
- 8.1. 개요
- 8.2. Introduction
- 8.3. 실험
- 8.4. 결과 및 논의
- 8.4.1. LFP, LTO
- 8.4.2. 15Wh 바이폴라 배터리
- 8.4.3. 안전성
- 8.5. 결론
9. 바이폴라 Ni-MH 배터리
- 9.1. 개요
- 9.2. 배터리 설계
- 9.3. 웨이퍼 셀 적용
- 9.4. HEV에의 적용
- 9.5. PHEV에의 적용
- 9.6. Utility에의 적용
- 9.6.1. 고출력 바이폴라 배터리
- 9.6.2. 고에너지 바이폴라 배터리
10. 바이폴라 고전압 Na-ion전지
- 10.1. 개요
- 10.2. Introduction
- 10.2.1. 모노폴라 48V 전지 시스템
- 10.2.2. 바이폴라 전지 상업화 이슈
- 10.3. 실험 및 방법
- 10.4. 결과
- 10.4.1. 액체 전해질 적용 바이폴라 전지
- 10.4.2. nS mP(직렬-병렬) 바이폴라 전지
- 10.4.3. 5V 이상 Na-ion 바이폴라 전지
- 10.4.4. 맞춤형 셀 전압 프로파일 설계
11. 바이폴라 전수지 전지(All polymer battery)
- 11.1. 전수지 전지의 특징
- 11.1.1. 전수지 전지의 장점
- 11.1.2. 전수지 전지의 단점
- 11.1.3. 전수지 전지의 에너지밀도
- 11.1.4. 전수지 전지 업체
- 11.1.4.1. 가와사키중공업
- 11.1.4.2. JFE 화학
- 11.1.4.3. Teijin
- 11.1.4.4. 군제/산요화성공업
- 11.2. 전수지 전지 제조방법
- 11.3. 전수지 전지 특성
- 11.3.1. 적층에 의한 전압 상승
- 11.3.2. 건조공정 불필요
- 11.3.3. 생산스피드 향상
- 11.4. 전수지 전지 기본 구조
- 11.5. 직렬 적층 바이폴라 전수지 전지
- 11.6. 안전성 향상된 바이폴라 전수지 전지
- 11.7. 코어 쉘형 전극 재료
- 11.8. APB사의 바이폴라 전수지 전지
- 11.9. 전수지 전지의 향후 전망
12. 바이폴라 납축전지(Furukawa電工)
- 12.1. 세계 최초의 실용화
- 12.1.1. 바이폴라형 축전지의 구조와 과제
- 12.1.2. 메탈/폴리머 소재를 통한 과제해결
- 12.2. 장주기용 전력 저장용 ESS 전지
13. 바이폴라 리튬전지(Fraunhofer IKTS)
- 13.1. 개요
- 13.2. 바이폴라 전지 개념
- 13.3. 습식공정 전극 제조
- 13.4. 분리막 코팅
- 13.5. 바이폴라 셀 및 스택
- 13.6. 바이폴라 전지용 roll clad foil(SCHLENK)
14. 바이폴라 Ni-MH 전지(TOYOTA)
- 14.1. 바이폴라 전지의 특징
- 14.2. 새로운 배터리 기술
- 14.3. TOYOTA의 배터리 혁신
- 14.4. TOYOTA의 타임라인
- 14.5. TOYOTA의 제조 프로세스
15. 바이폴라 전지 특허
- 15.1. TOYOTA: 바이폴라 Ni-MH 전지
- 15.2. 현대자동차: 바이폴라 전고체전지
- 15.3. TOYOTA: 바이폴라 전고체전지
- 15.4. SDI: 바이폴라 전극 및 제조
- 15.5. 엘지화학: 바이폴라 전지
- 15.6. LGES: 바이폴라 리튬이차전지
- 15.7. 한국생산기술연구원: 바이폴라 전고체 전지
- 15.8. ProLogium: 수평 복합 전기 공급 구조
A single-cell secondary battery consisted of monopolar electrodes, where both sides of the current collector are composed of the same electrode material, has all electrodes immersed in the same electrolyte. Since each electrode is connected in parallel using external connecting wires, a significant amount of inactive material has been integrated into the battery system. As a result, it is estimated that the volumetric energy density may experience a loss of approximately 40%, and the gravimetric energy density approximately 20%.
The bipolar battery features a simple cell configuration and shape as it does not utilize electrical connectors or other accessories. The volume of the battery is close to the product of the total stack thickness of the individual unit cells and the substrate area of the unit cell, while the weight of the battery is comparable to the total mass of all components. Although the capacity of the bipolar battery is equivalent to that of a single unit cell, the output voltage of the bipolar battery is determined by the number of unit cells connected in series and the voltage of each cell multiplied together.
Using bipolar electrodes in batteries significantly increases both volumetric and gravimetric energy density. Additionally, based on application-centric design, the battery shape can be easily adjusted to maximize the utilization of the battery storage space in the target device. In other words, the battery volume decreases, and by minimizing the BMS, energy density enhancement and cost savings can be simultaneously pursued through minimized use of cell packaging materials. This ultimately translates into the ability to install more batteries in limited electric vehicle battery mounting spaces, potentially leading to increased driving range. Therefore, these advantages of bipolar electrodes are highly attractive for the design of secondary batteries used in mobile electronic devices and electric vehicles.
Another advantage of bipolar electrodes is that electron flow occurs vertically through the substrate, and when the substrate's cross-sectional area is large, current density and distribution are significantly improved. Therefore, using bipolar electrodes allows fast-operating secondary batteries to function safely without any safety issues.
Starting with Furukawa Electric's compact batteries featuring bipolar electrodes, Toyota has recently commercialized bipolar Ni-MH batteries, which were applied to the Aqua HEV. In the announcement at June 2023, Toyota revealed a roadmap stating that they plan to produce bipolar LFP batteries for volume-grade EVs in 2026-2027 and bipolar Ni-based LIBs for future versions of EVs in 2027-2028. This roadmap aims to enhance driving range and reduce costs compared to performance versions of LIBs.
The recently released Toyota Crown Crossover and Lexus RX feature an improved version of the traditional Ni-MH battery, known as the bipolar Ni-MH. This marks a departure from the previous trend of using LIBs, especially in high-end and fuel-efficient models. This shift suggests an intention to gradually expand the use of Ni-MH batteries across the lineup, indicating a strategic change in battery technology adoption.
In this report, we have compiled the history of the development of bipolar electrodes, which have recently begun to be applied, as well as the current status of research and development. We have detailed each development to provide a comprehensive overview, making it easy to understand the overall situation.
The strong points of this report are as follows:
- 1. ailed coverage of recent technological trends related to bipolar batteries
- 2. ailed coverage of the development history and current status of bipolar battery developers
- 3. centrated coverage of the development status of bipolar batteries at Toyota Motor Corporation
- 4. lysis of bipolar battery's key patent
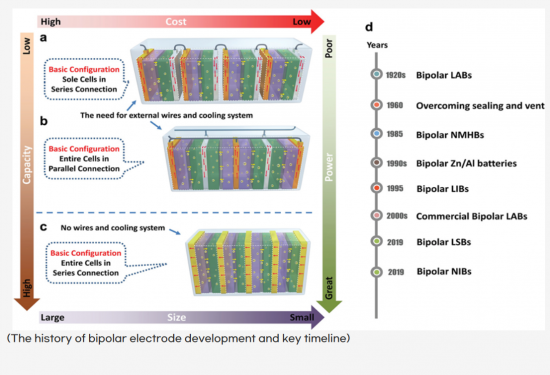
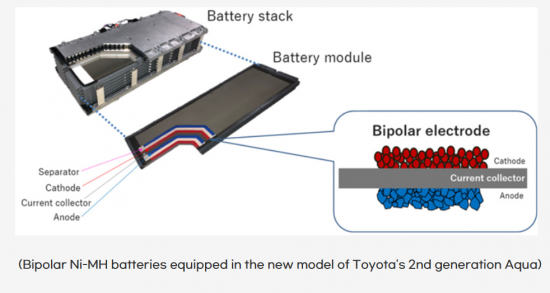
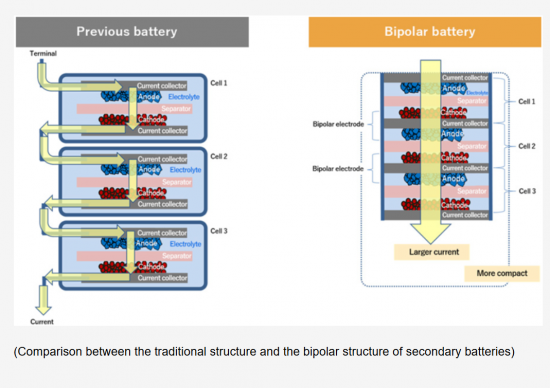
Table of Contents
1. Bipolar Electrodes for Secondary Batteries
- 1.1. The Necessity of Battery Structure Optimization
- 1.2. Bipolar Electrodes
- 1.3. Development of Bipolar Electrodes
- 1.3.1. History of Bipolar Electrode Development
- 1.3.2. Reduction in Weight, Size, and Cost
- 1.3.3. Improvement in Energy Density/Power Density
- 1.3.4. Requirements and Disadvantages of Bipolar Electrodes
- 1.4. Applications of Bipolar Electrodes
- 1.4.1. Bipolar Lead-Acid Batteries (LAB)
- 1.4.2. Improvement of Bipolar Lead-Acid Batteries
- 1.4.2.1. Surface Modification
- 1.4.2.2. Corrosion Prevention
- 1.4.3. Commercialization of Bipolar Lead-Acid Batteries
- 1.5. Bipolar Alkaline Batteries
- 1.5.1. Bipolar Ni-MH
- 1.5.2. Bipolar Al and Zn Batteries
- 1.6. Bipolar Lithium-Ion Batteries
- 1.7. Bipolar post-LiB(Li-S, Na-ion)
- 1.8. Challenges and Outlook
- 1.8.1. Substrate Materials
- 1.8.2. Electrode Materials
- 1.8.3. Electrolyte Materials
- 1.8.4. Engineering Technologies
- 1.8.5. Outlook of Bipolar Electrode
- 1.8.6. Hurdles to Commercialization
- 1.8.7. Other Bipolar Batteries
- 1.8.8. Bipolar Solid-State Batteries
2. Bipolar Solid-State Batteries: Design, Fabrication, and Electrochemistry
- 2.1. Overview
- 2.1.1. Advantages of Bipolar Solid-State Batteries
- 2.1.2. Technical Challenges of Bipolar Batteries
- 2.1.3. Requirements for Bipolar Materials
- 2.2. Bipolar Plates
- 2.3. Fabrication and Electrochemical Characteristics of Bipolar Solid-State Batteries
- 2.3.1. Free standing Lamination Bipolar Solid-State Batteries
- 2.3.2. Printing Bipolar Solid-State Batteries
- 2.4. Results and Future Outlook
3. Bipolar Solid-State Batteries: Design of Energy Density
- 3.1. Overview
- 3.2. Results and Discussion
- 3.2.1. SolidPAC demo
- 3.2.2. Comparison of Bipolar Stacking and Conventional Stacking
- 3.2.3. Sensitivity Analysis
- 3.2.4. Experimental Data Analysis
4. Bipolar Solid-State Batteries: Based on Quasi-Solid Electrolytes
- 4.1. Quasi-Solid Li-Glyme complex
- 4.2. Evaluation of Bipolar Solid-State LIBs
- 4.3. SEM Analysis of Bipolar Solid-State LIBs
- 4.4. Conclusion
5. Bipolar Solid-State Batteries: Based on Sulfide Electrolytes
- 5.1. Overview
- 5.2. Results and Discussion
- 5.2.1. Manufacturing and Characteristics of Cathode Layers
- 5.2.2. Manufacturing and Characteristics of Anode Layers
- 5.2.3. Manufacturing and Characteristics of Mono cells
- 5.2.4. Characteristics of Bipolar Stacked Solid-State Batteries
- 5.2.5. Comparison of Energy Densities in Bipolar Solid-State Batteries
- 5.3. Conclusion
6. Bipolar Solid-State Batteries: Based on Multistage Printing Manufacturing
- 6.1. Overview
- 6.2. Introduction
- 6.3. Experiment
- 6.3.1. Manufacturing of SWCNT-Coated Electrode Active Material
- 6.3.2. Fabrication of Printed Bipolar LIB
- 6.4. Results and Discussion
- 6.4.1. Solid Gel Composite Electrolyte(GCE)
- 6.4.2. Fabrication and Characteristics of Printed Electrodes
- 6.4.3. GCE and Electrode Paste Control
- 6.4.4. Mechanical Flexibility and Thermal Stability
- 6.5. Conclusion
7. Bipolar Solid-State Batteries: Application of FeOx-LFBO Anode
- 7.1. Overview
- 7.2. Introduction
- 7.3. Experiment Results
- 7.3.1. Synthesis and Characteristics of FeOx-LFBO
- 7.3.2. Electrochemical Performance of FeOx-LFBO Anode
- 7.3.3. Mechanism Analysis
- 7.3.4. Electrochemical Performance of Cu-free LIB
- 7.4. Conclusion
8. Bipolar LFP/LTO Batteries: LIBs for Micro/Mild Hybrid
- 8.1. Overview
- 8.2. Introduction
- 8.3. Experiments
- 8.4. Results and Discussion
- 8.4.1. LFP, LTO
- 8.4.2. 15Wh Bipolar Battery
- 8.4.3. Safety
- 8.5. Conclusion
9. Bipolar Ni-MH Batteries
- 9.1. Overview
- 9.2. Battery Design
- 9.3. Application of Wafer Cells
- 9.4. Application in HEVs
- 9.5. Application in PHEVs
- 9.6. Application in Utility
- 9.6.1. High-Power Bipolar Batteries
- 9.6.2. High-Energy Bipolar Batteries
10. Bipolar High-Voltage Na-ion Batteries
- 10.1. Overview
- 10.2. Introduction
- 10.2.1. Monopolar 48V Battery System
- 10.2.2. Commercialization Issues of Bipolar Batteries
- 10.3. Experiment and Method
- 10.4. Results
- 10.4.1. Bipolar Batteries with Liquid Electrolytes
- 10.4.2. nS mP(Series-Parallel) Bipolar Batteries
- 10.4.3. Na-ion Bipolar Batteries with Above 5V
- 10.4.4. Custom Cell Voltage Profile Design
11. Bipolar All Polymer Batteries
- 11.1. Characteristics of All Polymer Batteries
- 11.1.1. Advantages of All Polymer Batteries
- 11.1.2. Disadvantages of All Polymer Batteries
- 11.1.3. Energy Density of All Polymer Batteries
- 11.1.4. Manufacturers of All Polymer Batteries
- 11.1.4.1. Kawasaki Heavy Industries
- 11.1.4.2. JFE Chemical
- 11.1.4.3. Teijin
- 11.1.4.4. Gunze/Sanyo Chemical Industries
- 11.2. Manufacturing Methods of All Polymer Batteries
- 11.3. Characteristics of All Polymer Batteries
- 11.3.1. Voltage Increase by Stacking
- 11.3.2. Elimination of Drying Process
- 11.3.3. Improve of Production Speed
- 11.4. Basic Structure of All Polymer Batteries
- 11.5. All Polymer Batteries Stacked in Series
- 11.6. Safety-Enhanced Bipolar All Polymer Batteries
- 11.7. Core-Shell Type Electrode Materials
- 11.8. Bipolar All Polymer Batteries by APB Corporation
- 11.9. Future Outlook of All Polymer Batteries
12. Bipolar Lead-Acid Batteries (Furukawa Electric Co., Ltd.)
- 12.1. World's First Commercialization
- 12.1.1. Structure and Challenges of Bipolar Lead-Acid Batteries
- 12.1.2. Overcoming Challenges with Metal/Polymer Materials
- 12.2. ESS Batteries for Long-Term Power Storage
13. Bipolar LIBs (Fraunhofer IKTS)
- 13.1. Overview
- 13.2. Concept of Bipolar Batteries
- 13.3. Wet Process Electrode Manufacturing
- 13.4. Separator Coating
- 13.5. Bipolar Cells and Stacks
- 13.6. Roll Clad Foil for Bipolar Battery (SCHLENK)
14. Bipolar Ni-MH Batteries (TOYOTA)
- 14.1. Characteristics of Bipolar Batteries
- 14.2. New Battery Technologies
- 14.3. Battery Innovation by TOYOTA
- 14.4. Timeline of TOYOTA
- 14.5. Manufacturing Process by TOYOTA
15. Bipolar Battery Patents
- 15.1. TOYOTA: Bipolar Ni-MH Batteries
- 15.2. Hyundai Motor Company: Bipolar Solid-State Batteries
- 15.3. TOYOTA: Bipolar Solid-State Batteries
- 15.4. Samsung SDI: Bipolar Electrodes and Manufacturing
- 15.5. LG Chem: Bipolar Batteries
- 15.6. LGES: Bipolar LIBs
- 15.7. KITECH: Bipolar Solid-State Batteries
- 15.8. ProLogium: Horizontal Composite Electric Supply Structure
References
(주말 및 공휴일 제외)


















