|
연간정보 서비스
상품코드
1349829
HER2 저발현 전이성 유방암 시장 : Tumour DeckHER2-Low Metastatic Breast Cancer - Tumour Deck |
||||||
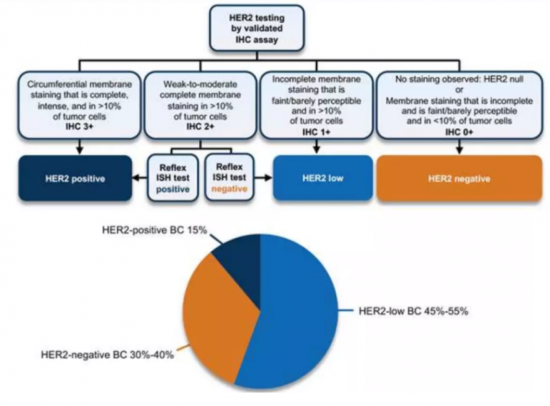
HER2 발현 수준이 낮고(HER2 IHC 스코어 1 또는 2로 정의), ERBB2 증폭이 검출되지 않는 종양은 이 HER2 저발현 전이성 유방암 범주에 속합니다. 이는 HER2 면역조직화학(IHC) 스코어 1 또는 스코어 2/in situ hybridization(ISH) 음성 표현형을 가진 HER2 음성 BC의 새롭게 정의된 하위 집합으로, IHC/ISH는 현재 HER2 발현을 정의하기 위해 적용되고 있는 유일한 표준 기술입니다. 입니다.
HER2 저발현 전이성 유방암 치료법은 빠르게 발전하고 있습니다. 최근 임상시험에서 CDK4/6 억제제와 내분비 요법을 병용하는 것이 표준 1차 치료로 효과적임이 입증되었습니다. 또한 PI3K 억제제와 AKT 억제제의 사용도 임상시험을 통해 검토되고 있으며, 가까운 시일 내에 더 많은 치료 옵션을 제공할 수 있을 것으로 보입니다.
HER2 저발현 전이성 유방암은 유방암의 새로운 아형으로 새로 진단된 유방암의 약 50%-60%를 차지합니다. 이는 HER2 저발현 전이성 유방암이 비교적 흔한 아형이며, HER2 저발현 유방암은 HER2 발현이 있더라도 일반적으로 HER2 음성으로 간주되어 치료되며, HER2 저발현은 HR 유방암에서 더 흔하지만, HR 음성 유방암에서도 발견된다는 연구 결과가 있습니다. 연구 결과에 따르면 HER2 저발현은 HR 유방암에서 더 흔하지만, HR 음성 유방암에서도
현재 HER2 저발현 전이성 유방암 치료의 주류는 화학요법, 내분비요법, 표적치료 등 다양한 치료법을 결합하는 것입니다.
최근 수년간 표적치료가 임상에서 유망한 치료법으로 떠오르면서 HER2 저치 전이성 유방암에 대한 현재의 표준치료는 최근 표적치료의 발전으로 빠르게 발전하고 있습니다. 최근 임상시험에서 새로운 HER2 지향성 항체-약물 복합체(ADC)가 HER2 저치환성 종양 치료에 큰 임상적 이점이 있는 것으로 입증되었습니다. 이러한 ADC 중 하나로 승인된 트루스투주맙-델크스테칸(T-Dxd)은 HER2 저발현 유방암에서 유망한 결과를 보여주고 있습니다.
표적치료 외에도 내분비요법은 HER2 저발현 유방암, 특히 호르몬 수용체 양성 환자에게 중요한 치료 옵션이며, CDK4/6 억제제와 내분비요법의 병용요법은 HER2 저발현 유방암 환자의 예후를 개선할 수 있는 유망한 치료법입니다.
세계의 HER2 저발현 전이성 유방암 시장에 대해 조사했으며, 시장 현황과 함께 증례수의 동향, 환자 동향, 경쟁 제품 시장 포지셔닝, 시장의 기회 등을 제공하고 있습니다.
목차
제1장 주요 요약
제2장 HER2 저발현 전이성 유방암의 개요
- HER2 저발현 전이성 유방암의 정의, 증상, 병인, 병인
- HER2-Low 현황의 임상적 의의
제3장 HER2 저발현 전이성 유방암의 정의와 진단
제4장 HER2 저발현 전이성 유방암의 역학
- 국가별 이환율
제5장 HER2 저발현 전이성 유방암의 치료 실천
- 현재의 치료법
- 치료 알고리즘
- 신속한 승인에 허용되는 엔드포인트
제6장 HER2 저발현 전이성 유방암 승인된 표적 치료
- 승인된 치료법의 개요
제7장 파이프라인 임상시험
- HER2 저발현 전이성 유방암 파이프라인 상황 개요와 분석
- HER2 저발현 전이성 유방암의 경쟁 구도
- 임상시험에서 주요 분자와 결과
- 주요 의약품 승인과 발매 타임라인
8장 제III상 자산
- 임상시험과 결과
제9장 제II상 자산
- 임상시험과 결과
제10장 제I상 자산
- 임상시험과 결과
제11장 HER2 저발현 전이성 유방암 파이프라인 비임상 분자
제12장 의사/KOL의 견해
- 미국, EU, 일본의 4명 KOL로부터의 인사이트
제13장 HER2 저발현 전이성 유방암의 주요 이벤트
- 승인된 표적치료의 확대
- 새로운 시행 가능한 목표 작성
제14장 HER2 저발현 전이성 유방암 시장 예측-2033년
- 주요 약제별 시장 예측과 환자 점유율
제15장 부록
KSA 23.09.27The current clinical definition of HER2-low Breast Cancer (HER2-Low BC) used in clinical practice and ongoing clinical trials relies on the standard IHC and ISH approach; thus, tumors with low level of HER2 expression (defined as a HER2 IHC score of 1+ or 2+) and no detectable ERBB2 amplification fall into this category. It is a newly defined subset of HER2-negative BC that has HER2 immunohistochemical (IHC) score of 1+ or score of 2+/in situ hybridization (ISH) negative phenotype. IHC/ISH is the only standard technique currently applied to define HER2 expression.
"The treatment armamentarium for HER2-low metastatic breast cancer is rapidly evolving. Recent clinical trials have demonstrated the efficacy of CDK4/6 inhibitors in combination with endocrine therapy as a standard first-line treatment option. Additionally, the use of PI3K inhibitors and AKT inhibitors is being explored in clinical trials and may provide further treatment options in the near future."
HER2 low metastatic breast cancer is a new subtype of breast cancer which accounts for approximately 50%-60% of newly diagnosed breast cancer cases. This indicates that HER2 low breast cancer is a relatively common subtype of the disease. Even though HER2-low breast cancer has some HER2 expression, it is generally considered and treated as HER2 negative. Studies have shown that HER2-low expression is more common in HR+ breast cancer, but it can also be found in HR negative breast cancer (Won et al., 2021; Tan et al., 2021).
Mellalta Meets HER2-Low Expression in Breast Cancer: Evaluating the Evidence, Challenges, and Opportunities for Expanding Treatment Benefit to More Patients
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Current Treatment Landscape
Currently the mainstay of treatment for HER2-Low Metastatic Breast Cancer consist of combination of different therapeutic approaches like chemotherapy, endocrine therapies, targeted therapies.
In recent years, targeted therapies have shown promise in clinical trials and are being explored as alternative treatment options. The current standard of care for HER2-low metastatic breast cancer is rapidly evolving due to recent advancements in targeted therapies. Recent clinical trials have demonstrated significant clinical benefits of novel HER2-directed antibody-drug conjugates (ADCs) in treating HER2-low tumors. One such approved ADC is trastuzumab deruxtecan (T-Dxd), which has shown promising results in HER2-low breast cancer.
In addition to targeted therapies, endocrine therapy is also an important treatment option for HER2-low breast cancer, particularly in patients with hormone receptor-positive disease. Combination therapies, such as CDK4/6 inhibitors in combination with endocrine therapy, have also shown promise in improving outcomes for patients with HER2-low breast cancer.
"It is exciting that we have been able to now translate HER2-targeted therapy to a broader group of patients with HER2-low-expressing breast cancer. Overall, promising responses to T-DXd offer newfound treatment possibilities for a substantial number of patients, many of whom were previously considered to have limited therapeutic options. The recognition of HER2-low status also signals an opportunity to develop more precise, individualized therapeutic approaches through future research."
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Current Unmet Needs
- Need for a clear and universally accepted definition of HER2-low, as the current classification is still evolving and there is ongoing research to determine the minimum threshold of HER2 expression required for treatment efficacy.
- HER2-low breast cancer (BC) has a poor prognosis, making the development of more suitable treatment an unmet clinical need.
- Need for standardized diagnostic criteria and guidelines for HER2-low tumors.
- Limited options for combination therapies that can enhance the efficacy of HER2-targeted treatments in HER2-low tumours.
"We are facing real challenges in terms of [HER2] identification in the clinic, and I would contend that we are in a state of flux in terms of the identification."
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Key Takeaways
- Human epidermal growth factor receptor 2 (HER2) breast cancer, especially in the unresected, metastatic setting, is no longer considered solely binary as positive or negative.
- There is a new generation of approved antibody drug conjugate (ADC) like Trastuzumab deruxtecan (T-DXd) that have a higher drug-to-antibody ratio (DAR) and can deliver the toxin in a more effective manner for advanced unresectable, metastatic breast cancer.
- The 2023 National Comprehensive Cancer Network (NCCN) guidelines for the use of trastuzumab deruxtecan (T-DXd) reflect the clinical trial eligibility for DESTINY-Breast04. This allows health care professionals to use other ADCs approved for hormone receptor (HR)-positive and triple negative disease, irrespective of HER2 status.
- A traditional immunohistochemical (IHC) assay and scoring system have been used in testing to identify HER2-low tumors and the tumors with an IHC score of more than 0, less than 1+. New technologies may help to better identify patients with this subtype of tumor.
- The recently presented data of the DAISY trial suggested meaningful activity of T-DXd even in patients with HER2-0 metastatic breast cancer
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Questions Answered:
- Potential challenges and opportunities in implementing targeted therapies for HER2-low breast cancer
- What is the size of clinically and commercially relevant drug-treatable HER2 low BC populations, and how will drug-treatment rates change over time?
- What is the expected market impact of recent drug approval such as Enhertu in treatment landscape of HER2-low metastatic BC?
- What are the most promising agents in the pipeline, and how will they shape the future of this therapy market?
- What key drivers and constraints will affect the HER2 low metastatic breast cancer therapy market over the forecast period?
Table of Content
1. Executive Summary
- 1.1. Summary of future trends
- 1.2. Potential opportunities to explore.
- 1.3. Drivers/barriers for entry
- 1.4. Unmet needs
- 1.5. What's new in HER2-Low Metastatic Breast Cancer?
2. HER2-Low Metastatic Breast Cancer Overview
- 2.1. HER2-Low Metastatic Breast Cancer definition, symptoms, etiology, Pathogenesis
- 2.2. Clinical Significance of HER2-Low Status
3. HER2-Low Metastatic Breast Cancer Definition & Diagnosis
- 3.1. Diagnostic Algorithm
- 3.2. HER2 Assessment with Immunohistochemistry (IHC) and In Situ Hybridization (ISH) (ASCO/CAP Guidelines)
- 3.3. AI-assisted interpretation of HER2 Status in HER2-Low Metastatic Breast Cancer
4. HER2-Low Metastatic Breast Cancer Epidemiology
- 4.1. Incidence rates by countries
5. HER2-Low Metastatic Breast Cancer Treatment Practices
- 5.1. Current treatment practices
- 5.2. Treatment algorithms
- 5.3. Acceptable endpoints for accelerated approval?
6. HER2-Low Metastatic Breast Cancer Approved Targeted Treatments
- 6.1. Quick overview of approved therapy
7. Pipeline clinical trials
- 7.1. HER2-Low Metastatic Breast Cancer pipeline landscape overview and analysis
- 7.2. Competitive Landscape of HER2-Low Metastatic Breast Cancer
- 7.3. Key molecules in clinical trials and results
- 7.4. Timeline of key drug approvals and launches.
8. Phase III Assets
- 8.1. Clinical trials and results
9. Phase II Assets
- 9.1. Clinical trials and results
10. Phase I Assets
- 10.1. Clinical trials and results
11. HER2-Low Metastatic Breast Cancer Pipeline Non-Clinical Molecules
- 11.1. Pre-clinical molecules
- 11.2. Mechanism of action, catalyst dates, and events
12. Physicians/KOLs Input
- 12.1. Insights from 4 KOLs in the US, EU, and Japan
13. Key Catalyst Events in HER2-Low Metastatic Breast Cancer
- 13.1. Expansion of approved targeted therapies
- 13.2. Creation of new actionable targets
14. HER2-Low Metastatic Breast Cancer Market Forecast -2033
- 14.1. Market Forecast and patient share by key drugs
15. Appendix
(주말 및 공휴일 제외)


















