
|
시장보고서
상품코드
1671847
자가 세포 치료 시장 : 제품 유형별, 용도별, 기술별, 최종사용자별, 지역별Autologous Cell Therapy Market, By Product Type, By Application, By Technology, By End User, By Geography |
||||||
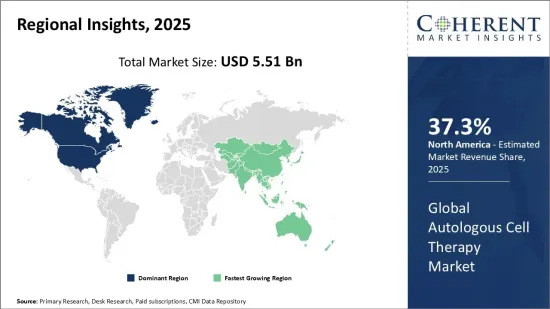
세계 자가 세포 치료 시장은 2025년에는 55억 1,000만 달러, 2032년에는 223억 달러에 달할 것으로 예상되며, 2025년부터 2032년까지 연평균 22.1%의 연평균 복합 성장률(CAGR)을 나타낼 것으로 예상됩니다.
| 보고 범위 | 보고서 상세 내용 | ||
|---|---|---|---|
| 기준 연도 | 2024년 | 2025년 시장 규모 | 55억 1,000만 달러 |
| 실적 데이터 | 2020-2024년 | 예측 기간 | 2025-2032년 |
| 예측 기간(2025-2032년) CAGR | 22.1% | 2032년 가치 예측 | 223억 달러 |
세계 자가 세포 치료 시장은 최근 몇 년동안 괄목할 만한 성장세를 보이고 있습니다. 자가 세포 치료는 환자 자신의 몸에서 줄기세포나 다른 유형의 세포를 채취하여 다양한 과정을 통해 변형 또는 증식시킨 후 동일한 환자에게 주입하여 질병을 치료하는 것입니다. 암이나 신경질환과 같은 복잡한 질병을 면역원성 우려를 최소화하면서 치료할 수 있는 대안적 치료 접근법을 제공합니다. 만성 질환의 유병률 증가, 개인 맞춤형 의료에 대한 수요 증가, 동종 세포에 비해 자가 세포의 우월성 등이 향후 자가 세포 치료 수요를 촉진할 것으로 예상되는 주요 요인입니다. 그러나 시장 개발 및 제조에 따른 높은 비용과 규제상의 문제가 시장 진입에 큰 장벽으로 작용하고 있습니다.
시장 역학:
세계 자가 세포 치료 시장은 만성질환에 취약한 노인 인구 증가, 제품 개발을 촉진하기 위한 공공 및 민간 기업의 투자 증가, 개인 맞춤형 치료 옵션에 대한 수요 증가에 힘입어 성장하고 있습니다. 그러나 높은 개발 비용, 복잡한 제조 공정, 엄격한 규제 정책은 예측 기간 동안 시장 성장을 억제할 것으로 예상됩니다. 새로운 적응증에 대한 자가 세포 치료제의 유효성과 안전성을 평가하기 위한 다양한 임상시험이 진행 중이며, 혁신적인 제조 기술 개발은 기존 기업뿐만 아니라 신규 진출기업들에게도 큰 비즈니스 기회를 제공합니다. 주요 기업들은 이러한 치료법의 적용을 확대하기 위해 학술 연구 기관과 협력하고 있습니다. 아시아태평양은 중국, 인도 등 주요 국가의 헬스케어 인프라 성장과 헬스케어 지출 증가로 인해 유리한 시장 기회를 제공합니다.
본 조사의 주요 특징
세계의 자가 세포 치료 시장에 대해 조사 분석했으며, 2024년을 기준 연도로 하여 예측기간(2025-2032년) 시장 규모와 연평균 성장률(CAGR)에 대해 조사 분석하여 전해드립니다.
또한, 다양한 부문에 걸친 잠재적 수익 기회를 밝히고, 이 시장의 매력적인 투자 제안 매트릭스를 설명합니다.
또한 시장 성장 촉진요인, 억제요인, 기회, 신제품 출시 및 승인, 시장 동향, 지역별 전망, 주요 기업의 경쟁 전략 등에 대한 주요 고찰을 제공합니다.
이 보고서는 기업 하이라이트, 제품 포트폴리오, 주요 하이라이트, 재무 성과, 전략 등의 매개 변수를 기반으로 세계 자가 세포 치료 시장의 주요 기업을 프로파일링합니다.
주요 기업으로는 Bristol Myers Squibb, Novartis, Novartis, Gilead Sciences, Kite Pharma, Celgene, Amgen, Takeda Pharmaceutical Industry, Merck KGaA, Bluebird Bio, Celyad Oncology, Adaptimmune Therapeutics, Allogene Therapeutics, Athersys, Inc. 등이 있습니다.
이 보고서의 통찰력을 통해 마케팅 담당자와 기업 경영진은 향후 제품 출시, 유형화, 시장 확대, 마케팅 전술에 대한 정보에 입각한 의사결정을 내릴 수 있습니다.
이 보고서는 투자자, 공급업체, 제품 제조업체, 유통업체, 신규 시장 진출기업, 재무 분석가 등 업계의 다양한 이해관계자를 대상으로 합니다.
이해관계자들은 세계 자가 세포 치료 시장 분석에 사용되는 다양한 전략 매트릭스를 통해 의사결정을 쉽게 내릴 수 있습니다.
목차
제1장 조사 목적과 전제조건
- 조사 목적
- 전제조건
- 약어
제2장 시장 전망
- 보고서 설명
- 시장 정의와 범위
- 주요 요약
제3장 시장 역학, 규제, 동향 분석
- 시장 역학
- 성장 촉진요인
- 성장 억제요인
- 기회
- 영향 분석
- 주요 발전
- 규제 시나리오
- 제품 발매/승인
- PEST 분석
- PORTER 분석
- 인수합병(M&A) 시나리오
- 업계 동향
제4장 세계의 자가 세포 치료 시장, 제품 유형별, 2020년-2032년
- 세포 치료
- 조직 치료
- 유전자 치료
제5장 세계의 자가 세포 치료 시장, 용도별, 2020년-2032년
- 종양학
- 심혈관 질환
- 신경 질환
- 정형외과 질환
- 자가면역 질환
- 창상 치유
제6장 세계의 자가 세포 치료 시장, 기술별, 2020년-2032년
- 줄기세포 요법
- CAR T 세포치료
- T세포 수용체(TCR) 요법
- 유도만능 줄기세포(iPSC)
제7장 세계의 자가 세포 치료 시장, 최종사용자별, 2020년-2032년
- 병원
- 연구기관
- 바이오테크놀러지 기업
- 제약회사
- 기타
제8장 세계의 자가 세포 치료 시장, 지역별, 2020년-2032년
- 북미
- 라틴아메리카
- 유럽
- 아시아태평양
- 중동
- 아프리카
제9장 경쟁 구도
- Bristol Myers Squibb
- Novartis
- Gilead Sciences
- Kite Pharma
- Celgene
- Amgen
- Takeda Pharmaceutical Company
- Merck KGaA
- Bluebird Bio
- Celyad Oncology
- Adaptimmune Therapeutics
- Allogene Therapeutics
- Athersys, Inc.
- BrainStorm Cell Therapeutics
- Sorrento Therapeutics
제10장 애널리스트 추천 사항
- Wheel of Fortune
- 애널리스트의 견해
- COM(Coherent Opportunity Map)
제11장 참고 문헌과 조사 방법
- 참고 문헌
- 조사 방법
- 출판사에 대해
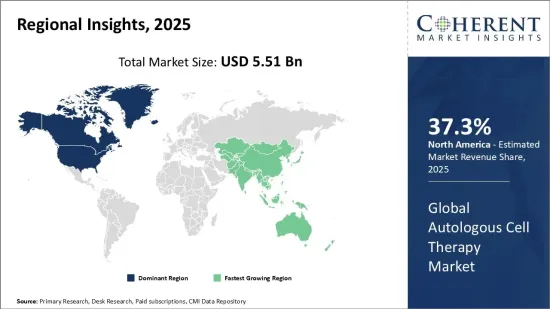
The Global Autologous Cell Therapy Market is estimated to be valued at USD 5.51 Bn in 2025 and is expected to reach USD 22.30 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 22.1% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 5.51 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 22.10% | 2032 Value Projection: | USD 22.30 Bn |
The global autologous cell therapy market has been witnessing significant growth in the recent years. Autologous cell therapy involves the collection of stem cells or other types of cells from patient's own body, modifying or expanding them through various processes and infusing back to the same patient to treat diseases. It provides an alternative therapeutic approach to treat complex diseases like cancer and neurological disorders with minimal immunogenicity concerns. Rising prevalence of chronic diseases, growing demand for personalized medicine, and advantages of autologous cells over allogeneic cells are some key factors expected to propel demand for autologous cell therapies going forward. However, high costs associated with development and manufacturing along with regulatory challenges are major barriers to the market.
Market Dynamics:
The global autologous cell therapy market is driven by rising geriatric population vulnerable to develop chronic diseases, increasing investments from public and private players to expedite product development, and growing demand for personalized treatment options. However, high development costs, complex manufacturing processes, and stringent regulatory policies are projected to restrain the market growth during the forecast period. Various ongoing clinical trials evaluating efficacy and safety of autologous cell therapies for new indications and development of innovative manufacturing technologies present significant opportunities for existing as well as new market players. Key players are collaborating with academic research institutions to expand applications of these therapies. Asia Pacific presents lucrative market opportunities with growing healthcare infrastructure and increasing healthcare spending in major countries like China and India.
Key Features of the Study:
This report provides in-depth analysis of the global autologous cell therapy market, and provides market size (US$ Billion) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approvals, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global autologous cell therapy market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Bristol Myers Squibb, Novartis, Gilead Sciences, Kite Pharma, Celgene, Amgen, Takeda Pharmaceutical Company, Merck KGaA, Bluebird Bio, Celyad Oncology, Adaptimmune Therapeutics, Allogene Therapeutics, Athersys, Inc., BrainStorm Cell Therapeutics, and Sorrento Therapeutics
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
The global autologous cell therapy market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global autologous cell therapy market
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2020 - 2032)
- Cell-based therapies
- Tissue-based therapies
- Gene therapies
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Oncology
- Cardiovascular diseases
- Neurological disorders
- Orthopedic disorders
- Autoimmune diseases
- Wound healing
- Technology Insights (Revenue, USD Bn, 2020 - 2032)
- Stem cell therapy
- CAR T-cell therapy
- T-cell receptor (TCR) therapy
- Induced pluripotent stem cells (iPSCs)
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals
- Research institutes
- Biotechnology companies
- Pharmaceutical companies
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Bristol Myers Squibb
- Novartis
- Gilead Sciences
- Kite Pharma
- Celgene
- Amgen
- Takeda Pharmaceutical Company
- Merck KGaA
- Bluebird Bio
- Celyad Oncology
- Adaptimmune Therapeutics
- Allogene Therapeutics
- Athersys, Inc.
- BrainStorm Cell Therapeutics
- Sorrento Therapeutics
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Global Autologous Cell Therapy Market, By Product Type
- Global Autologous Cell Therapy Market, By Application
- Global Autologous Cell Therapy Market, By Technology
- Global Autologous Cell Therapy Market, By End User
- Global Autologous Cell Therapy Market, By Region
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Driver
- Restraint
- Opportunity
- Impact Analysis
- Key Developments
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Industry Trends
4. Global Autologous Cell Therapy Market, By Product Type, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Cell-based therapies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Tissue-based therapies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Gene therapies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
5. Global Autologous Cell Therapy Market, By Application, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Oncology
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Cardiovascular diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Neurological disorders
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Orthopedic disorders
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Autoimmune diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Wound healing
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
6. Global Autologous Cell Therapy Market, By Technology, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Stem cell therapy
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- CAR T-cell therapy
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- T-cell receptor (TCR) therapy
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Induced pluripotent stem cells (iPSCs)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
7. Global Autologous Cell Therapy Market, By End User, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Research institutes
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Biotechnology companies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Pharmaceutical companies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
8. Global Autologous Cell Therapy Market, By Region, 2020 - 2032, Value (USD Bn)
- Introduction
- Market Share (%) Analysis, 2025, 2028 & 2032, Value (USD Bn)
- Market Y-o-Y Growth Analysis (%), 2021 - 2032, Value (USD Bn)
- Regional Trends
- North America
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Technology, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- U.S.
- Canada
- Latin America
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Technology, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Technology, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Technology, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Technology, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, By Product Type, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Application, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Technology, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By End User, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country/Region, 2020 - 2032, Value (USD Bn)
- South Africa
- North Africa
- Central Africa
9. Competitive Landscape
- Bristol Myers Squibb
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Novartis
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Gilead Sciences
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Kite Pharma
- Celgene
- Amgen
- Takeda Pharmaceutical Company
- Merck KGaA
- Bluebird Bio
- Celyad Oncology
- Adaptimmune Therapeutics
- Allogene Therapeutics
- Athersys, Inc.
- BrainStorm Cell Therapeutics
- Sorrento Therapeutics
10. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
11. References and Research Methodology
- References
- Research Methodology
- About us
















