
|
시장보고서
상품코드
1578764
베트남의 인간용 백신 수입(2024-2033년)Vietnam Human Vaccines Import Research Report 2024-2033 |
||||||
인간용 백신은 수년간 예방 효과를 발휘하여 인간의 건강에 필수적인 역할을 하고 있습니다. 현대 생활에서 예방 접종은 특정 질병, 특히 전염병과 감염으로부터 예방하는 가장 효과적인 방법으로 널리 사람들에게 받아 들여지고 있습니다.
인포그래픽스
베트남 총 인구는 2023년 말 1억 명을 돌파하며 매년 100만 명 이상의 순인구가 증가하고 있습니다. 경제가 발전하고 자본소득이 증가함에 따라 베트남의 의약품 산업은 강력하게 성장하고 있습니다. 또한 베트남 정부는 지원 정책을 지속적으로 내세우고 의료 지출을 확대하고 있습니다. CRI에 따르면 베트남의 자유 무역 협정은 제약 산업에서 외국 기업 시장 진입 장벽을 없애고 유럽과 일부 지역의 의약품 수입 관세는 매우 낮거나 존재하지 않을 수도 있습니다. 베트남의 제약 산업은 수입 의약품에 크게 의존하고 있습니다.
백신은 특정 질병을 예방하고 질병의 확산을 억제하며 공중 보건을 향상시킵니다. CRI는 베트남에서 인간용 백신 수요가 꾸준히 증가하고 있다고 분석했습니다. 그러나 국내 생산 능력이 제한되어 있기 때문에 베트남의 인간용 백신 시장은 주로 수입 제품에 의존합니다. 최근 베트남은 COVID-19 백신, 인플루엔자 백신, HPV 백신, 4가 및 6가 소아 백신 등 수많은 백신을 수입하고 있습니다. 베트남의 백신 수요는 높고, 특히 COVID-19 이후에는 정부나 국민 모두 건강을 중시하게 되어, 수입되는 인간용 백신의 수가 증가하고 있습니다.
백신 산업에는 원료 공급(항원, 보조제 등), 제조(연구, 제조, 품질 관리 등)와 같은 업스트림 부문과 유통 및 예방접종 서비스 등 다운스트림 부문이 있습니다. 백신 제조에는 엄격한 위생 조건, 고급 제조 설비 및 기술, 높은 수준의 품질 관리가 필요합니다. 세계의 주요 백신 제조 및 수출 기업은 Pfizer, Modernna, AstraZeneca 등 강력한 연구개발능력을 지닌 선도적인 바이오 의약품 기업이며, 현재 베트남 국내 기업은 한정된 유형의 백신만 제조할 수 있습니다.
COVID-19에서 베트남은 긴급 수요에 부응하기 위해 상당수의 백신을 수입했습니다. CRI에 따르면 베트남에서는 인간용 백신 제조의 인프라와 기술이 발달되지 않았기 때문에 공급은 국제 시장에 크게 의존하고 있습니다. 또한 베트남 정부는 공중 보건에 매우 힘을 쏟고 있으며 일반적인 감염을 다루는 국가 예방 접종 프로그램(NIP)을 통해 백신 접종률 향상을 추진하고 있습니다. 베트남은 백신의 안정적인 공급을 보장하기 위해 다양한 국제적 백신 공급업체와 협력 협정을 맺고 있습니다.
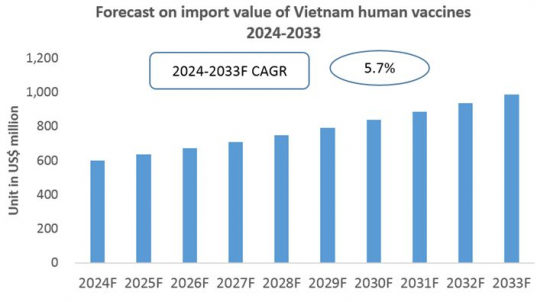
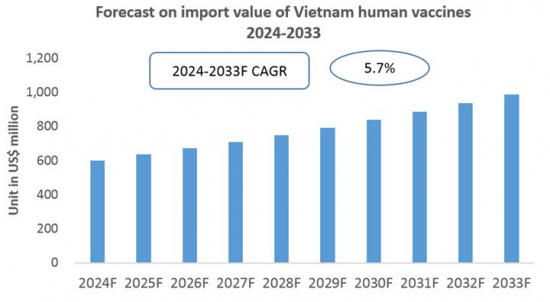
CRI 데이터에 따르면 베트남의 인간용 백신 수입 총액은 2023년 4억 7,000만 달러에 달했습니다. 또한 2024년 1월부터 5월까지 베트남 백신 수입 총액은 2억 5,000만 달러를 넘어 전년 대비 30% 이상의 성장세를 보였습니다. 이 추세는 앞으로 수년간 안정적인 성장률로 지속될 것으로 예상됩니다.
전반적으로 베트남 인구 증가와 경제 수준 상승 외에도 건강에 대한 의식의 고조에 따라 베트남 인간용 백신 시장 규모가 확대될 전망입니다. CRI 분석에 따르면 국산 인간용 백신의 생산 능력과 기술 수준이 비교적 낮기 때문에 베트남의 인간용 백신 수입은 향후 수년간 지속될 것으로 예상됩니다.
본 보고서에서는 베트남의 인간용 백신 수입 동향을 조사하여 국가 개요, 수입액, 수입량 및 수입 가격 등의 추이 및 예측, 수입원 상위 국가별 상세 분석, 주요 바이어 및 공급자 분석, 목제 가구 수출에 대한 주요 영향요인 분석 등을 요약합니다.
목차
제1장 베트남 개요
- 지역
- 경제 상황
- 인구통계
- 국내 시장
- 인간용 백신 수입 시장에 참가하는 외국 기업에 대한 권고
제2장 베트남의 인간용 백신 수입의 분석(2021-2024년)
- 수입규모
- 수입액 및 수입량
- 수입가격
- 소비량
- 수입 의존도
- 인간용 백신의 주요 수입원
제3장 베트남의 인간용 백신의 주요 수입원 분석(2021-2024년)
- 미국
- 수입량 및 수입액의 분석
- 평균 수입 가격 분석
- 프랑스
- 수입량 및 수입액의 분석
- 평균 수입 가격 분석
- 벨기에
- 수입량 및 수입액의 분석
- 평균 수입 가격 분석
- 태국
- 네덜란드
- 인도
제4장 베트남의 인간용 백신 수입 시장의 주요 공급자 분석(2021-2024년)
- GLAXOSMITHKLINE BIOLOGICALS SA
- MERCK SHARP & DOHME(ASIA) LTD
- SANOFI PASTEUR
- 기타
- 기업 개요
- 인간용 백신 수출 분석
제5장 베트남의 인간용 백신 수입 시장의 주요 수입업체 분석(2021-2024년)
- GSK PHARMA VIETNAM COMPANY
- MSD HH VIETNAM LTD
- SANOFI-AVENTIS VIETNAM COMPANY
- 기타
- 기업 개요
- 인간용 백신 수입의 분석
제6장 베트남의 인간용 백신 수입의 월별 분석(2021-2024년)
- 월별 수입액 및 수입량의 분석
- 월평균 수입가격 예측
제7장 베트남의 인간용 백신 수입에 영향을 주는 주요 요인
- 정책
- 현재 수입정책
- 수입 정책의 동향 예측
- 경제
- 시장 가격
- 인간용 백신 생산 능력의 성장 동향
- 기술
제8장 베트남에서의 인간용 백신 수입 예측
면책사항
서비스 보증
AJY 24.11.04Human vaccines offer longstanding protection, playing an indispensable role in human health. In modern life, vaccination has become the most effective way to prevent from specific diseases, especially pestilences and infection, widely accepted by people.
INFOGRAPHICS
By the end of 2023, Vietnam's total population has surpassed 100 million, with a net population growth of more than 1 million annually. As the economy progressing and capital income increasing, the pharmaceutical industry in Vietnam is growing vigorously. Also, the Vietnam government continues to issue support policies and expand healthcare spending. For example, the national strategy for pharmaceutical industry development through 2030, with a vision to 2045 approved in 2023 set out clear development targets for specific industry including vaccines. According to CRI, Vietnam's free trade agreements have removed market access barriers for foreign companies in its pharmaceutical industry, and import tariffs on medicines from Europe and some regions are very low or even non-existent. Vietnam's pharmaceutical industry is highly dependent on imported pharmaceutical products.
Vaccines can prevent certain diseases, reduce the spread of illness, and improve public health. CRI has analyzed that the demand for human vaccines in Vietnam is steadily increasing. However, due to limited domestic production capacity, Vietnam's human vaccine market mainly relies on imported products. In recent years, Vietnam has imported a large number of vaccines, including COVID-19 vaccines, influenza vaccines, HPV vaccines, and quadrivalent and hexavalent pediatric vaccines. The demand for vaccines in Vietnam is high, especially in the post-COVID-19 era, as both the government and the public place greater emphasis on health, leading to an increase in the number of imported human vaccines.
The vaccine industry involves upstream sectors such as raw material supply (e.g., antigens, adjuvants), manufacturing (including research, production, and quality control), and downstream sectors like distribution and vaccination services. Vaccine production requires strict hygiene conditions, advanced production equipment and technology, and high standards of quality control. Leading global vaccine producers and exporters are typically large biopharmaceutical companies with strong R&D capabilities, such as Pfizer, Moderna, and AstraZeneca. Currently, Vietnamese domestic companies are only able to produce a limited variety of vaccines.
During the COVID-19 pandemic, Vietnam imported a significant number of vaccines to meet the urgent demand. According to CRI, because of the underdeveloped infrastructure and technology for human vaccine production in Vietnam, the country is heavily reliant on the international market for supply. Moreover, the Vietnamese government is highly committed to public health, promoting higher vaccination rates through the National Immunization Program (NIP), which covers a range of common infectious diseases. Vietnam has entered into cooperation agreements with various international vaccine suppliers to ensure a stable supply of vaccines.
Based on data from CRI, in 2023, Vietnam's total imports of human vaccines reached USD 470 million. From January to May 2024, Vietnam's total vaccine imports exceeded USD 250 million, representing a year-on-year growth of over 30%. This trend is expected to continue at a steady growth rate in the coming years.
CRI's analysis reveals that from 2021 to 2024, Vietnam's primary sources of human vaccine imports were the United States, France, and Belgium. Major companies exporting human vaccines to Vietnam include GlaxoSmithKline Biologicals S.A., Merck Sharp & Dohme (Asia) Ltd., and Sanofi Pasteur.
The main importers of human vaccines in Vietnam are pharmaceutical companies, distributors, and logistics providers, mostly foreign enterprises, such as GSK Pharma Vietnam Company, MSD HH Vietnam Ltd., and Sanofi-Aventis Vietnam Company.
Overall, with Vietnam's growing population and rising economic level, coupled with increased awareness of health, the size of Vietnam's human vaccine market is set to expand. CRI's analysis indicates that due to the relatively low production capacity and technological levels of domestic human vaccines, Vietnam's human vaccine imports are expected to continue growing in the coming years.
Topics covered:
The Import and Export of Human Vaccines in Vietnam (2021-2024)
Total Import Volume and Percentage Change of Human Vaccines in Vietnam (2021-2024)
Total Import Value and Percentage Change of Human Vaccines in Vietnam (2021-2024)
Total Import Volume and Percentage Change of Human Vaccines in Vietnam (2024)
Total Import Value and Percentage Change of Human Vaccines in Vietnam (2024)
Average Import Price of Human Vaccines in Vietnam (2021-2024)
Top 10 Sources of Human Vaccines Imports in Vietnam and Their Supply Volume
Top 10 Suppliers in the Import Market of Human Vaccines in Vietnam and Their Supply Volume
Top 10 Importers of Human Vaccines in Vietnam and Their Import Volume
How to Find Distributors and End Users of Human Vaccines in Vietnam
How Foreign Enterprises Enter the Human Vaccines Market of Vietnam
Forecast for the Import of Human Vaccines in Vietnam (2024-2033)
Table of Contents
1 Overview of Vietnam
- 1.1 Geography of Vietnam
- 1.2 Economic Condition of Vietnam
- 1.3 Demographics of Vietnam
- 1.4 Domestic Market of Vietnam
- 1.5 Recommendations for Foreign Enterprises Entering the Vietnam Human Vaccines Imports Market
2 Analysis of Human Vaccines Imports in Vietnam (2021-2024)
- 2.1 Import Scale of Human Vaccines in Vietnam
- 2.1.1 Import Value and Volume of Human Vaccines in Vietnam
- 2.1.2 Import Prices of Human Vaccines in Vietnam
- 2.1.3 Apparent Consumption of Human Vaccines in Vietnam
- 2.1.4 Import Dependency of Human Vaccines in Vietnam
- 2.2 Major Sources of Human Vaccines Imports in Vietnam
3 ANALYSIS OF MAJOR SOURCES OF HUMAN VACCINES IMPORTS IN VIETNAM (2021-2024)
- 3.1 United States
- 3.1.1 Analysis of Vietnam's Human Vaccines Import Volume and Value from the United States
- 3.1.2 Analysis of Average Import Price
- 3.2 France
- 3.2.1 Analysis of Vietnam's Human Vaccines Import Volume and Value from France
- 3.2.2 Analysis of Average Import Price
- 3.3 Belgium
- 3.3.1 Analysis of Vietnam's Human Vaccines Import Volume and Value from Belgium
- 3.3.2 Analysis of Average Import Price
- 3.4 Thailand
- 3.5 Netherlands
- 3.6 India
4 Analysis of Major Suppliers in the Import Market of Human Vaccines in Vietnam (2021-2024)
- 4.1 GLAXOSMITHKLINE BIOLOGICALS S A
- 4.1.1 Company Introduction
- 4.1.2 Analysis of Human Vaccines Exports to Vietnam
- 4.2 MERCK SHARP & DOHME (ASIA) LTD
- 4.2.1 Company Introduction
- 4.2.2 Analysis of Human Vaccines Exports to Vietnam
- 4.3 SANOFI PASTEUR
- 4.3.1 Company Introduction
- 4.3.2 Analysis of Human Vaccines Exports to Vietnam
- 4.4 Exporter 4
- 4.4.1 Company Introduction
- 4.4.2 Analysis of Human Vaccines Exports to Vietnam
- 4.5 Exporter 5
- 4.5.1 Company Introduction
- 4.5.2 Analysis of Human Vaccines Exports to Vietnam
- 4.6 Exporter 6
- 4.6.1 Company Introduction
- 4.6.2 Analysis of Human Vaccines Exports to Vietnam
- 4.7 Exporter 7
- 4.7.1 Company Introduction
- 4.7.2 Analysis of Human Vaccines Exports to Vietnam
- 4.8 Exporter 8
- 4.8.1 Company Introduction
- 4.8.2 Analysis of Human Vaccines Exports to Vietnam
- 4.9 Exporter 9
- 4.9.1 Company Introduction
- 4.9.2 Analysis of Human Vaccines Exports to Vietnam
- 4.10 Exporter 10
- 4.10.1 Company Introduction
- 4.10.2 Analysis of Human Vaccines Exports to Vietnam
5 Analysis of Major Importers in the Import Market of Human Vaccines in Vietnam (2021-2024)
- 5.1 GSK PHARMA VIETNAM COMPANY
- 5.1.1 Company Introduction
- 5.1.2 Analysis of Human Vaccines Imports
- 5.2 MSD HH VIETNAM LTD
- 5.2.1 Company Introduction
- 5.2.2 Analysis of Human Vaccines Imports
- 5.3 SANOFI-AVENTIS VIETNAM COMPANY
- 5.3.1 Company Introduction
- 5.3.2 Analysis of Human Vaccines Imports
- 5.4 Importer 4
- 5.4.1 Company Introduction
- 5.4.2 Analysis of Human Vaccines Imports
- 5.5 Importer 5
- 5.5.1 Company Introduction
- 5.5.2 Analysis of Human Vaccines Imports
- 5.6 Importer 6
- 5.6.1 Company Introduction
- 5.6.2 Analysis of Human Vaccines Imports
- 5.7 Importer 7
- 5.7.1 Company Introduction
- 5.7.2 Analysis of Human Vaccines Imports
- 5.8 Importer 8
- 5.8.1 Company Introduction
- 5.8.2 Analysis of Human Vaccines Imports
- 5.9 Importer 9
- 5.9.1 Company Introduction
- 5.9.2 Analysis of Human Vaccines Imports
- 5.10 Importer 10
- 5.10.1 Company Introduction
- 5.10.2 Analysis of Human Vaccines Imports
6. Monthly Analysis of Human Vaccines Imports in Vietnam from 2021 to 2024
- 6.1 Analysis of Monthly Import Value and Volume
- 6.2 Forecast of Monthly Average Import Prices
7. Key Factors Affecting Human Vaccines Imports in Vietnam
- 7.1 Policy
- 7.1.1 Current Import Policies
- 7.1.2 Trend Predictions for Import Policies
- 7.2 Economic
- 7.2.1 Market Prices
- 7.2.2 Growth Trends of Human Vaccines Production Capacity in Vietnam
- 7.3 Technology
8. Forecast for the Import of Human Vaccines in Vietnam, 2024-2033
Disclaimer
Service Guarantees



















