
|
시장보고서
상품코드
1885914
의료 규제 업무 아웃소싱 시장 : 기회, 성장 요인, 업계 동향 분석 및 예측(2025-2034년)Healthcare Regulatory Affairs Outsourcing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
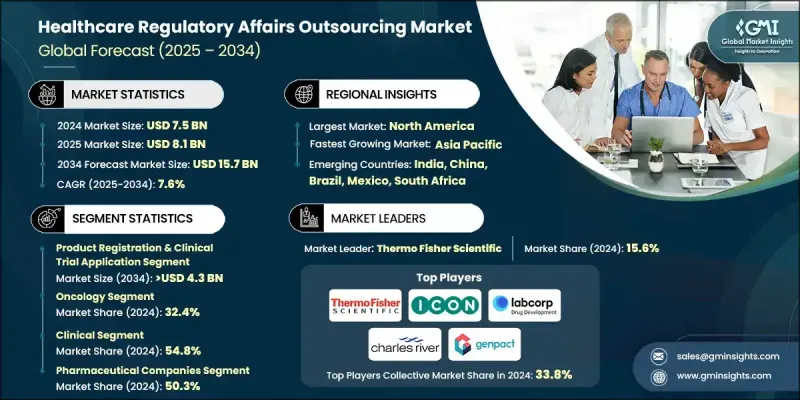
세계의 의료 규제 업무 아웃소싱 시장은 2024년에 75억 달러로 평가되었고, 2034년까지 연평균 복합 성장률(CAGR)은 7.6%로 성장해 157억 달러에 달할 것으로 예측되고 있습니다.
세계의 규제 프레임워크의 복잡성 증가로 제약, 바이오기술, 의료기기 기업들은 규제 준수, 문서화 및 제출 업무를 전문 파트너사에 아웃소싱하고 있습니다. 해당 시장은 규제 공정 간소화, 국제 표준 준수 보장, 제품 승인 가속화를 지원하는 종단간 솔루션을 제공합니다. 서비스에는 규제 전략 수립, 서류 준비, eCTD 발행, 라벨링 관리, 라이프사이클 유지보수, 규제 인텔리전스 등이 포함되며, 모두 효율성, 안전성 및 환자 결과 개선을 목표로 합니다. 디지털 및 클라우드 기반 규제 정보 관리(RIM) 시스템의 급속한 도입과 AI 기반 분석 기술의 결합은 전자 제출 및 복잡한 규제 워크플로우 관리에 능숙한 아웃소싱 제공업체에 대한 수요를 촉진하고 있습니다. 또한 적응형 및 분산형 연구를 포함한 글로벌 임상시험의 증가로 규제 부담이 커지면서 기업들은 제출 계획 수립, 규제 준수 추적, 보건 당국과의 상호작용을 위해 경험 많은 외부 파트너에 의존하고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 기간 | 2025-2034년 |
| 시작 금액 | 75억 달러 |
| 예측 금액 | 157억 달러 |
| CAGR | 7.6% |
2024년 기준 제품 등록 및 임상시험 신청 부문이 28.4%의 점유율을 차지했습니다. 이 부문의 성장은 전 세계적으로 증가하는 의약품 개발 활동과 엄격한 규제 요건에 힘입었습니다. 의약품 안전성과 효능을 보호하기 위해 FDA, EMA, PMDA와 같은 기관의 지침이 지속적으로 강화됨에 따라, 제출 절차의 복잡성을 헤쳐 나가고 규제 준수를 보장하기 위해서는 아웃소싱이 필수적입니다.
2024년 종양학 부문은 32.4%의 점유율을 차지했으며, 2025-2034년 동안 54억 달러에 달할 것으로 예상됩니다. 전 세계적으로 증가하는 암 발병률, 인식 제고 및 조기 발견 프로그램이 혁신적인 종양학 치료법에 대한 수요를 촉진하고 있습니다. 기업들은 종양학 약물 개발에 막대한 투자를 하고 있어 복잡한 다지역 규제 제출을 초래하고 있습니다. 종양학 임상시험의 높은 비용과 긴 기간은 종양학 규제에 특화된 전문성을 보유한 기업에 대한 아웃소싱을 더욱 장려합니다.
북미의 의료 규제 업무 아웃소싱 시장은 2024년 46.5%의 점유율을 차지했습니다. 이 지역의 우위는 강력한 제약, 바이오기술 및 의료기기 산업에서 비롯되며, 이들은 엄격한 규제 프레임워크 준수를 위해 광범위한 규제 지원이 필요합니다. 미국 FDA 및 캐나다 보건부(Health Canada)와 같은 당국의 진화하는 규제(임상 시험, 생물학적 제제, 시판 후 모니터링에 관한 지침 포함)는 아웃소싱 파트너에 대한 의존도를 높였습니다.
자주 묻는 질문
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 규제 요건 준수 필요성 증가
- 혁신 의약품 및 의료기기에 대한 신속 승인 절차 수요 급증
- 임상시험 건수 증가
- 규제 복잡성 증가
- 업계의 잠재적 위험 및 과제
- 데이터 보안 및 개인정보 보호에 대한 우려
- 표준화의 부족
- 시장 기회
- 신흥 시장을 위한 규제 아웃소싱 확대
- 엔드 투 엔드 RIM(규제 정보 관리)에 대한 수요 증가
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 북미
- 유럽
- 아시아태평양
- LAMEA(중동 및 아프리카)
- 기술 동향
- 현재의 기술 동향
- 클라우드 기반 규제 제출 및 문서 관리 플랫폼 도입
- 규제 인텔리전스 및 규제 준수 모니터링을 위한 AI 및 머신러닝 도입
- 세계 제출 상황 추적 및 전자 제출을 위한 디지털 툴 통합
- 신흥기술
- AI를 활용한 규제 의사결정 지원 및 예측 규제 준수 분석
- 안전하고 투명성이 높고 추적 가능한 규제 문서를 위한 블록체인
- 현재의 기술 동향
- 장래 시장 동향
- 시장 진입을 가속시키는 규제상의 이정표
- 연구개발 및 투자활동 증가
- 강화된 규제 준수와 밸리데이션
- 투자 및 자금 조달 환경
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업 매트릭스 분석
- 기업의 시장 점유율 분석
- 세계
- 북미
- 유럽
- 아시아태평양
- 경쟁 포지셔닝 매트릭스
- 주요 시장 기업의 경쟁 분석
- 주요 발전
- 합병 및 인수
- 제휴 및 협업
- 신제품 발매
- 확대 계획
제5장 시장 추계 및 예측 : 서비스별(2021-2034년)
- 주요 동향
- 제품 등록 및 임상시험 신청
- 규제 컨설팅/전략적 서비스
- 신청 서류 관리
- 법적 대리 업무
- 규제 문서 작성 및 출판
- 기타 서비스
제6장 시장 추계 및 예측 : 적응증별(2021-2034년)
- 주요 동향
- 종양학
- 신경학
- 순환기학
- 면역학
- 기타 적응증
제7장 시장 추계 및 예측 : 제품 개발 단계별(2021-2034년)
- 주요 동향
- 비임상 단계
- 임상 단계
- 시판 후 승인(PMA)
제8장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 제약기업
- 생명공학 기업
- 의료기기 제조업체
제9장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Accell Clinical Research
- Charles River Laboratories
- Clinilabs
- Freyr
- Genpact
- ICON
- Labcorp
- NAMSA
- PAREXEL
- PharmaLex
- ProPharma
- Proventa
- Syneos Health
- Thermo Fisher Scientific
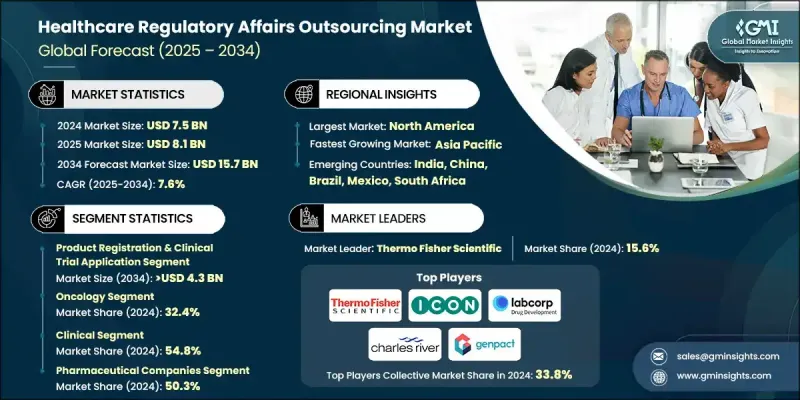
The Global Healthcare Regulatory Affairs Outsourcing Market was valued at USD 7.5 billion in 2024 and is estimated to grow at a CAGR of 7.6% to reach USD 15.7 billion by 2034.
The increasing complexity of global regulatory frameworks compelling pharmaceutical, biotechnology, and medical device companies to outsource compliance, documentation, and submission tasks to specialized partners. The market provides end-to-end solutions that help streamline regulatory processes, ensure adherence to international standards, and accelerate product approvals. Services include regulatory strategy formulation, dossier preparation, eCTD publishing, labeling management, lifecycle maintenance, and regulatory intelligence, all aimed at enhancing efficiency, safety, and patient outcomes. The rapid adoption of digital and cloud-based regulatory information management (RIM) systems, combined with AI-driven analytics, is driving demand for outsourcing providers skilled in managing electronic submissions and complex regulatory workflows. Furthermore, the growing number of global clinical trials, including adaptive and decentralized studies, is increasing regulatory burdens and prompting companies to rely on experienced external partners for submission planning, compliance tracking, and interactions with health authorities.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $7.5 Billion |
| Forecast Value | $15.7 Billion |
| CAGR | 7.6% |
In 2024, the product registration & clinical trial application segment held a 28.4% share. This segment's growth is fueled by increasing drug development activities and stringent regulatory requirements worldwide. Outsourcing is essential to navigate the complexity of submissions, ensuring compliance with agencies like the FDA, EMA, and PMDA, which continue to tighten guidelines to safeguard drug safety and efficacy.
The oncology segment held a 32.4% share in 2024 and is expected to reach USD 5.4 billion during 2025-2034. Rising global cancer incidence, increasing awareness, and early detection programs are driving demand for innovative oncology therapies. Companies are investing heavily in oncology drug development, creating complex, multi-region regulatory submissions. The high cost and long duration of oncology trials further encourage outsourcing to firms with specialized expertise in oncology regulations.
North America Healthcare Regulatory Affairs Outsourcing Market held a 46.5% share in 2024. The region's dominance stems from its robust pharmaceutical, biotechnology, and medical device industries, which require extensive regulatory support to comply with stringent frameworks. Evolving regulations from authorities such as the U.S. FDA and Health Canada, including guidelines on clinical trials, biologics, and post-market monitoring, have increased reliance on outsourcing partners.
Key players operating in the Global Healthcare Regulatory Affairs Outsourcing Market include Thermo Fisher Scientific, Clinilabs, Genpact, ICON, Syneos Health, Accell Clinical Research, Charles River Laboratories, Labcorp, NAMSA, PAREXEL, PharmaLex, ProPharma, and Proventa. Companies in the Healthcare Regulatory Affairs Outsourcing Market strengthen their presence by investing in specialized expertise, particularly for complex therapy areas like oncology and biologics. They develop integrated digital platforms for electronic submissions, AI-driven regulatory intelligence, and cloud-based RIM systems to streamline compliance workflows. Strategic partnerships with global pharmaceutical and biotech firms help expand service portfolios and geographic reach. Firms focus on regulatory consulting, dossier management, and lifecycle maintenance to enhance customer loyalty.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Services trends
- 2.2.3 Indication trends
- 2.2.4 Product stage trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing need to comply with regulatory requirements
- 3.2.1.2 Surging demand for faster approval process for breakthrough drugs and devices
- 3.2.1.3 Rising number of clinical trials
- 3.2.1.4 Increasing regulatory complexity
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Data security and privacy concerns
- 3.2.2.2 Lack of standardization
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of regulatory outsourcing for emerging markets
- 3.2.3.2 Rising demand for end-to-end RIM
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 LAMEA
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.1.1 Adoption of cloud-based regulatory submission and document management platforms
- 3.5.1.2 Implementation of AI and machine learning for regulatory intelligence and compliance monitoring
- 3.5.1.3 Integration of digital tools for global submission tracking and e-submissions
- 3.5.2 Emerging technologies
- 3.5.2.1 AI-enabled regulatory decision support and predictive compliance analytics
- 3.5.2.2 Blockchain for secure, transparent, and traceable regulatory documentation
- 3.5.1 Current technological trends
- 3.6 Future market trends
- 3.6.1 Regulatory milestone accelerating market entry
- 3.6.2 Increased R&D and investment activity
- 3.6.3 Enhanced regulatory compliance and validation
- 3.7 Investment and funding landscape
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Services, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Product registration & clinical trial application
- 5.3 Regulatory consulting/strategic services
- 5.4 Submission management
- 5.5 Legal representation
- 5.6 Regulatory writing & publishing
- 5.7 Other services
Chapter 6 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Neurology
- 6.4 Cardiology
- 6.5 Immunology
- 6.6 Other indications
Chapter 7 Market Estimates and Forecast, By Product Stage, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Preclinical
- 7.3 Clinical
- 7.4 Post market authorization (PMA)
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pharmaceutical companies
- 8.3 Biotechnology companies
- 8.4 Medical device companies
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Accell Clinical Research
- 10.2 Charles River Laboratories
- 10.3 Clinilabs
- 10.4 Freyr
- 10.5 Genpact
- 10.6 ICON
- 10.7 Labcorp
- 10.8 NAMSA
- 10.9 PAREXEL
- 10.10 PharmaLex
- 10.11 ProPharma
- 10.12 Proventa
- 10.13 Syneos Health
- 10.14 Thermo Fisher Scientific














