
|
시장보고서
상품코드
1876793
면역치료제 시장 : 시장 기회, 성장 요인, 업계 동향 분석 및 예측(2025-2034년)Immunotherapy Drugs Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
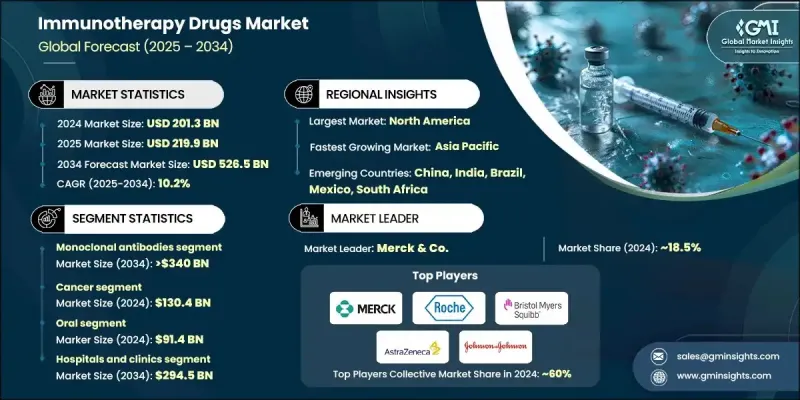
세계의 면역치료제 시장은 2024년에 2,013억 달러로 평가되었고, 2034년까지 연평균 복합 성장률(CAGR) 10.2%로 성장할 전망이며, 5,265억 달러에 이를 것으로 예측되고 있습니다.

시장 확대의 배경에는 암, 자가면역질환, 감염증 등 만성질환의 유병률 상승에 더해 항체 공학에 있어서 지속적인 기술 혁신이 영향을 주고 있습니다. CAR-T 세포 요법, 이중특이적 항체, 면역관문억제제와 같은 고급 치료 기술은 기존의 치료법보다 우수한 효능과 안전성을 제공하고 치료 패러다임을 변화시키고 있습니다. 인공지능은 창약을 가속화하고 바이오마커를 기반으로 한 임상 개발은 환자 선택과 치료 성공률을 향상시킵니다. 유전자 프로파일 및 분자 프로파일을 바탕으로 설계된 치료법이 세계적으로 받아들여지고 있는 가운데 정밀의료 및 개별화 의료로의 지속적인 이행이 시장 성장을 더욱 강화하고 있습니다. 새로운 항원 기반 백신 및 바이오마커를 기반으로 하는 치료 계획과 같은 새로운 접근법은 모니터링 및 치료 성과를 향상시키는 유전체학, 단백질체학, 진단 도구의 진보에 힘입어 급속히 발전하고 있습니다. 면역치료제는 항체 및 단백질과 같은 생물 공학적으로 설계된 물질을 사용하여 면역 활성화 또는 억제 메커니즘을 통해 면역 반응을 수정하거나 조절하고 신체가 다양한 질병과 싸울 수 있도록 설계되었습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 기간 | 2025-2034년 |
| 시작 시 가치 | 2,013억 달러 |
| 예측 금액 | 5,265억 달러 |
| CAGR | 10.2% |
단일클론항체 부문은 2024년에 63.3%의 점유율을 차지하였고, 2034년까지 3,400억 달러에 이를 전망이며, CAGR 10.4%로 성장할 것으로 예측됩니다. 만성 질환의 발병률 상승은 표적형 생물학적 제형에 대한 강한 수요를 지속적으로 견인하고 있습니다. 단일클론항체는 기존의 의약품에 비해 우수한 선택성 및 부작용의 저감으로 치료의 기반이 되고 있습니다. 특정 분자 경로를 표적으로 하는 능력은 현대 의료에서 필수적인 존재로 삼아 세계적인 면역치료 개발에서 가장 가치 있는 구성요소 중 하나로서의 지위를 확립하고 있습니다.
암 치료 분야는 2024년에 1,304억 달러 시장 규모를 만들어 냈으며, 세계 면역치료제 시장에서 주요 치료 영역으로서의 지위를 유지하고 있습니다. 세계적인 암 증례의 급증과 선진적 생물학적 요법의 지속적인 진화가 시장 확대의 핵심적인 요인이 되고 있습니다. 면역관문억제제, 단일클론항체, 차세대 세포 요법과 같은 혁신적인 치료법은 환자의 생존율과 연주 효율을 향상시켜 종양학 치료의 개념을 재정의했습니다. 자가면역질환 분야도 급속한 성장을 기록하고 있으며, 전신적 면역억제에서 정밀표적형 생물학적 요법으로의 초점 이동으로 질병 관리의 개선 및 부작용의 저감을 실현하고 있습니다.
미국의 면역치료제 시장은 2024년에 831억 달러에 이르렀으며, 연구개발 및 임상 혁신에 있어서 세계적인 주도적 입장을 강화하고 있습니다. 바이오테크놀러지 기반 스타트업 기업, 제약 대기업, 강력한 연방정부 지원으로 이루어진 이 나라의 생태계는 신규 면역치료의 신속한 상업화를 가능하게 하고 있습니다. Amgen, Pfizer, and Johnson & Johnson 등 기업의 지속적인 투자로 차세대 생물학적 및 세포 요법의 파이프라인이 확대되고 있으며, 수많은 임상시험의 승인이 면역치료의 진보 가속을 위한 동국의 대처를 부각하고 있습니다.
세계 면역 요법 약물 시장의 주요 시장 진출 기업으로는 Bristol Myers Squibb, AstraZeneca, Gilead Sciences, F. Hoffmann La Roche, Johnson & Johnson, Novartis, GlaxoSmithKline, Sanofi, Merck & Co., Moderna, Pfizer, Amgen, Kite Pharma, Adaptimmune Therapeutics 및 Bluebird Bio 등이 포함됩니다. 세계 면역치료제 시장의 주요 기업은 세계적인 존재감 강화 및 경쟁력 향상을 위한 다양한 전략을 전개하고 있습니다. 신규 면역경로를 표적으로 한 연구개발 프로그램의 확충과 이중 특이성 항체나 CAR-T 플랫폼 등 차세대 바이오로직에 대한 투자를 추진하고 있습니다. 전략적 제휴, 합병, 인수를 통해 첨단 기술에 대한 액세스를 확보하고 임상 개발의 타임라인을 가속시키고 있습니다. 또한 선진 치료에 대한 액세스 확대를 도모하기 위해 특히 신흥 경제국에서 지역적인 사업 확대에도 주력하고 있습니다.
자주 묻는 질문
목차
제1장 조사 방법 및 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 공급자의 상황
- 각 단계에서의 부가가치
- 밸류체인에 영향을 주는 요인
- 업계에 미치는 영향요인
- 성장 촉진요인
- 만성 질환 증가 경향
- 항체 공학에서의 기술적 진보
- 개별화 및 표적 요법에 대한 수요 증가
- 비종양학 영역에 대한 용도 확대
- 업계의 잠재적 위험 및 과제
- 높은 비용 및 제한된 접근 가능성
- 환자의 반응 및 내성의 변동성
- 시장 기회
- 분산형 제조 및 지역 허브
- 개별화 네오 항원 백신
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 북미
- 유럽
- 아시아태평양
- 기술 동향
- 현재의 기술 동향
- 신흥 기술
- 장래 시장 동향
- 가격 분석
- 임상시험 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서문
- 기업의 시장 점유율 분석
- 세계
- 북미
- 유럽
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 주요 발전
- 합병 및 인수
- 제휴 및 협력 관계
- 신제품 발매
- 확대 계획
제5장 시장 추계 및 예측 : 약제 유형별(2021-2034년)
- 주요 동향
- 단일클론항체
- 백신
- 인터페론 α 및 β
- 인터루킨
- 기타 약제 유형
제6장 시장 추계 및 예측 : 용도별(2021-2034년)
- 주요 동향
- 암
- 자가면역질환
- 감염증
- 기타 용도
제7장 시장 추계 및 예측 : 투여 경로별(2021-2034년)
- 주요 동향
- 정맥내 투여
- 피하 투여
- 경구
제8장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원 및 진료소
- 암 연구 기관
- 제약 및 바이오테크놀러지 기업
- 기타 최종 용도
제9장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Adaptimmune Therapeutics
- Amgen
- AstraZeneca
- Bristol Myers Squibb
- Bluebird Bio
- F. Hoffmann La Roche
- GlaxoSmithKline
- Gilead Sciences
- Johnson & Johnson
- Kite Pharma
- Merck & Co.
- Moderna
- Novartis
- Pfizer
- Sanofi
The Global Immunotherapy Drugs Market was valued at USD 201.3 billion in 2024 and is estimated to grow at a CAGR of 10.2% to reach USD 526.5 billion by 2034.
The market expansion is influenced by the rising prevalence of chronic illnesses, including cancer, autoimmune, and infectious diseases, coupled with continuous innovation in antibody engineering. Advanced therapeutic technologies such as CAR-T cell therapy, bispecific antibodies, and immune checkpoint inhibitors are transforming treatment paradigms, offering improved efficacy and safety over conventional therapies. Artificial intelligence is accelerating drug discovery, while biomarker-driven clinical development is enhancing patient selection and treatment success rates. The ongoing shift toward precision and personalized medicine continues to strengthen market growth, as therapies designed around genetic and molecular profiles gain global acceptance. Emerging approaches such as neoantigen-based vaccines and biomarker-guided regimens are advancing rapidly, supported by progress in genomics, proteomics, and diagnostic tools that improve monitoring and outcomes. Immunotherapy drugs are designed to modify or regulate immune responses, helping the body combat various diseases through either immune activation or suppression mechanisms using biologically engineered substances such as antibodies or proteins.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $201.3 Billion |
| Forecast Value | $526.5 Billion |
| CAGR | 10.2% |
The monoclonal antibodies segment accounted for 63.3% share in 2024 and is expected to reach USD 340 billion by 2034, growing at a CAGR of 10.4%. The rising incidence of chronic conditions continues to drive strong demand for targeted biologics. Monoclonal antibodies have become a cornerstone of treatment due to their superior selectivity and reduced side effects compared to traditional pharmaceuticals. Their ability to target specific molecular pathways has made them indispensable in modern medical therapies and positioned them as one of the most valuable components of immunotherapy development worldwide.
The cancer segment generated USD 130.4 billion in 2024, maintaining its position as the leading therapeutic area within the global immunotherapy drugs market. The surge in global cancer cases and the ongoing evolution of advanced biological therapies are central to market expansion. Innovative treatments, including immune checkpoint modulators, monoclonal antibodies, and next-generation cell therapies, have redefined oncology care by improving patient survival and response rates. The autoimmune disease segment is also recording rapid growth as the focus shifts from generalized immune suppression to precision-targeted biologic therapies, resulting in better disease management and fewer adverse effects.
U.S. Immunotherapy Drugs Market reached USD 83.1 billion in 2024, reinforcing its global leadership position in research, development, and clinical innovation. The country's ecosystem of biotechnology startups, pharmaceutical giants, and strong federal support enables rapid commercialization of novel immunotherapies. Ongoing investments by companies such as Amgen, Pfizer, and Johnson & Johnson are expanding the pipeline of next-generation biologics and cellular therapies, with numerous investigational new drug approvals highlighting the nation's commitment to accelerating immunotherapy advancements.
Major participants in the Global Immunotherapy Drugs Market include Bristol Myers Squibb, AstraZeneca, Gilead Sciences, F. Hoffmann La Roche, Johnson & Johnson, Novartis, GlaxoSmithKline, Sanofi, Merck & Co., Moderna, Pfizer, Amgen, Kite Pharma, Adaptimmune Therapeutics, and Bluebird Bio. Key companies in the Global Immunotherapy Drugs Market are employing diverse strategies to enhance their global presence and strengthen competitiveness. They are expanding R&D programs targeting novel immune pathways and investing in next-generation biologics such as bispecific antibodies and CAR-T platforms. Strategic alliances, mergers, and acquisitions are enabling them to access cutting-edge technologies and accelerate clinical development timelines. Firms are also focusing on regional expansion, particularly in emerging economies, to increase access to advanced treatments.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Drug type trends
- 2.2.3 Application trends
- 2.2.4 Route of administration trends
- 2.2.5 End Use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of chronic diseases
- 3.2.1.2 Technological advancement in antibody engineering
- 3.2.1.3 Growing demand for personalized and targeted therapies
- 3.2.1.4 Expansion into non-oncology applications
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost and limited accessibility
- 3.2.2.2 Variable patient response and resistance
- 3.2.3 Market opportunities
- 3.2.3.1 Decentralized manufacturing and regional hubs
- 3.2.3.2 Personalized neoantigen vaccines
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Future market trends
- 3.7 Pricing analysis
- 3.8 Clinical trial analysis
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Drug Type, 2021 - 2034 ($ Bn)
- 5.1 Key trends
- 5.2 Monoclonal antibodies
- 5.3 Vaccines
- 5.4 Interferons alpha & beta
- 5.5 Interleukins
- 5.6 Other drug types
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Bn)
- 6.1 Key trends
- 6.2 Cancer
- 6.3 Autoimmune diseases
- 6.4 Infectious diseases
- 6.5 Other applications
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 - 2034 ($ Bn)
- 7.1 Key trends
- 7.2 Intravenous
- 7.3 Subcutaneous
- 7.4 Oral
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Bn)
- 8.1 Key trends
- 8.2 Hospitals and clinics
- 8.3 Cancer research institutes
- 8.4 Pharmaceutical and biotechnology companies
- 8.5 Other End Use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Bn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Adaptimmune Therapeutics
- 10.2 Amgen
- 10.3 AstraZeneca
- 10.4 Bristol Myers Squibb
- 10.5 Bluebird Bio
- 10.6 F. Hoffmann La Roche
- 10.7 GlaxoSmithKline
- 10.8 Gilead Sciences
- 10.9 Johnson & Johnson
- 10.10 Kite Pharma
- 10.11 Merck & Co.
- 10.12 Moderna
- 10.13 Novartis
- 10.14 Pfizer
- 10.15 Sanofi



















