
|
시장보고서
상품코드
1833641
자궁내 피임기구(IUD) 시장 : 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Intrauterine Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
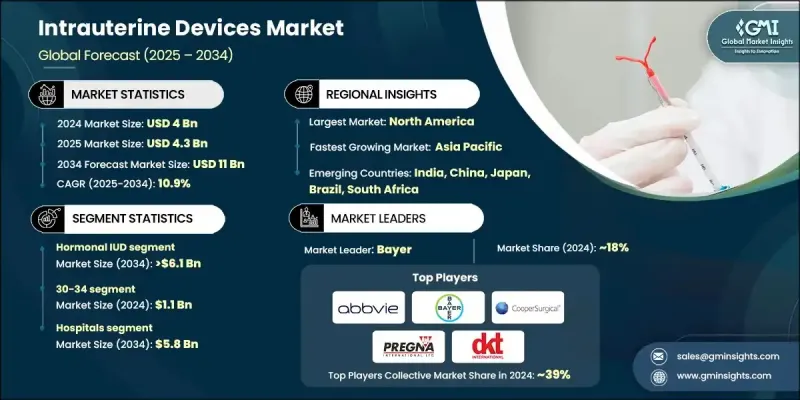
세계의 자궁내 피임기구 시장은 2024년에는 40억 달러로 평가되었고, CAGR 10.9%로 성장할 전망이며, 2034년에는 110억 달러에 이를 것으로 추정됩니다.

이 강력한 성장 궤도의 원동력이 되는 것은 지지적인 규제 정책, IUD의 다양한 사용법에 관한 여성 의식 고조, 의도치 않은 임신의 높은 발생률입니다. IUD는 피임의 역할뿐만 아니라 월경 다량 출혈, 자궁 내막증, 갱년기 장애의 관리 등, 보다 폭넓은 건강에 대한 용도가 인정되고 있습니다. 교육적 노력과 디지털 헬스 플랫폼 상승, 리프로덕티브 케어 정보에 대한 접근성 향상으로 여성들은 장기적인 피임 옵션에 대한 더 많은 정보를 얻고 의사 결정을 내릴 수 있게 되었습니다. 헬스케어 전문가는 IUD의 안전, 효능 및 기타 건강 혜택에 대해 환자에게 상담을 함으로써 중요한 역할을 하고 있으며, 이는 세계적인 수용을 뒷받침하고 있습니다. 자궁내 피임기구는 작고 유연한 T 자형 피임기구로 신뢰할 수 있고 가역적이며 장시간 작용하는 임신 예방을 제공하도록 설계되었습니다. 철을 주성분으로 하는 비호르몬 피임구와 같은 새로운 기술 혁신은 부작용을 최소화하고 호르몬을 사용하지 않는 솔루션을 제공하기 위해 개발되었습니다. 이러한 기술적 진보는 IUD의 전망을 바꾸고 여성의 선택을 늘리고 장기적인 시장 확대를 강화하고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시장 규모 | 40억 달러 |
| 예측 금액 | 110억 달러 |
| CAGR | 10.9% |
호르몬성 IUD 부문은 2024년에 53.8%의 점유율을 차지했으며, 임신을 예방하고 월경의 건강을 개선하는 효과에 지지되고 있습니다. 호르몬 IUD, 특히 레보놀게스트렐을 방출하는 IUD는 장기간의 보호, 낮은 유지보수, 월경 출혈 감소 및 자궁내막증 증상의 완화와 같은 치료상의 이점이 있기 때문에 널리 채택되고 있습니다. 의료 서비스 제공업체는 이중 장점으로 인해 자주 이를 권장하며 시장에서 우위를 강화하고 있습니다.
병원 부문은 2024년에 53.2%의 점유율을 차지했으며, 2034년까지 58억 달러를 창출할 것으로 예측됩니다. 병원은 숙련된 부인과 의사와 산과 의사가 안전한 삽입 절차를 수행할 수 있는 접근을 제공하기 때문에 장기간 작용하는 가역적 피임법을 제공하는 데 필수적인 존재로 남아 있습니다. 여러 지역에서 산후 IUD 삽입 프로토콜을 채택함으로써 접근이 확대되고 특히 임산부 헬스케어 시스템이 확고한 지역에서는 예기치 않은 임신 감소에 기여합니다.
미국 자궁내 피임기구 2024년 시장 규모는 14억 4,000만 달러로, 2025-2034년 연평균 복합 성장률(CAGR) 10.3%로 성장할 것으로 추정됩니다. 공중 보건에 대한 노력과 계몽 활동의 활성화로 안전하고 효과적이며 편리한 피임법으로 IUD를 사용하는 것이 좋습니다. 매일 알약과 같은 단기 대체품보다 오랫동안 작용하는 가역적 피임에 대한 선호도가 높아짐에 따라 미국과 캐나다의 다양한 인구 역학에서 채택이 더욱 가속화되고 있습니다.
자궁내 피임기구 업계에서 활약하는 유명한 기업으로는 MONA LISA, Meril, Sebela Pharmaceuticals, DKT, PREGNA, SMB, AbbVie, Prosan, GIMA, Bayer, Mediines 360, eurogine, GYNO CARE, CooperSurgical, HLL Lifecare Limited 등이 있습니다. 자궁내 피임기구 시장에서 발판을 굳히기 위해 주요 기업은 부작용을 최소화하고 사용감을 향상시키기 위해 고안된 혁신적인 소재 개발을 포함하여 호르몬과 비 호르몬의 선택을 가진 제품 포트폴리오의 확대에 주력하고 있습니다. 헬스케어 조직 및 정부 프로그램과의 전략적 제휴는 접근 및 채용률을 확대하기 위해 진행되고 있습니다. 또한 월경 헬스케어 등 피임 이외의 IUD의 이점에 대해 여성을 교육하는 계발 캠페인에도 많은 기업이 투자하고 있습니다.
목차
제1장 조사 방법 및 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 바람직한 규제 시나리오
- 다양한 IUD의 적용에 관한 여성 의식의 고조
- 원하지 않는 임신이 많음
- 원치 않는 낙태 및 임신을 막기 위한 정부의 이니셔티브
- 계획적인 임신의 연기에 대한 경향 증가
- 업계의 잠재적 위험 및 과제
- 디바이스의 고비용
- 일부 건강 문제의 위험
- 보험의 적용 범위 및 액세스의 편차
- 시장 기회
- 장기 피임 수요 증가
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술적 상황
- 현재의 기술
- 신흥 기술
- 장래 시장 동향
- 환급 시나리오
- 소비자 행동 및 동향
- 브랜드 분석
- 파이프라인 분석
- 피임 이외의 치료에 대한 용도
- 가격 분석(2024년)
- Porter's Five Forces 분석
- PESTEL 분석
- 갭 분석
제4장 경쟁 구도
- 서문
- 기업 매트릭스 분석
- 기업의 시장 점유율 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 주요 발전
- 합병 및 인수
- 파트너십 및 협업
- 신제품 발매
- 확장 계획
제5장 시장 추계 및 예측 : 제품별(2021-2034년)
- 주요 동향
- 구리 부착 IUD
- 호르몬 성 자궁 내 피임기구
제6장 시장 추계 및 예측 : 연령별(2021-2034년)
- 주요 동향
- 15-19세
- 20-24세
- 25-29세
- 30-34세
- 35-39
- 40-44세
- 45세 이상
제7장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원
- 부인과 클리닉
- 지역 의료 센터
제8장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제9장 기업 프로파일
- AbbVie
- Bayer
- CooperSurgical
- DKT
- eurogine
- GIMA
- GYNO CARE
- HLL Lifecare Limited
- Medicines360
- Meril
- MONA LISA
- PREGNA
- Prosan
- Sebela Pharmaceuticals
- SMB
The Global Intrauterine Devices Market was valued at USD 4 billion in 2024 and is estimated to grow at a CAGR of 10.9% to reach USD 11 billion by 2034.
The strong growth trajectory is driven by supportive regulatory policies, greater awareness among women about the diverse uses of IUDs, and the high incidence of unintended pregnancies. IUDs are increasingly acknowledged not only for their role in birth control but also for wider health applications, including the management of heavy menstrual bleeding, endometriosis, and perimenopausal symptoms. With the rise of educational initiatives, digital health platforms, and improved access to reproductive care information, women are now empowered to make more informed decisions about long-term contraceptive options. Healthcare professionals play a significant role by counseling patients on the safety, effectiveness, and additional health benefits of IUDs, which has boosted acceptance globally. Intrauterine devices are small, flexible, T-shaped contraceptive tools designed to provide reliable, reversible, and long-acting pregnancy prevention. Emerging innovations, such as iron-based non-hormonal devices, are being developed to minimize side effects and provide hormone-free solutions. These technological advancements are transforming the IUD landscape, enhancing choices for women, and reinforcing long-term market expansion.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4 Billion |
| Forecast Value | $11 Billion |
| CAGR | 10.9% |
The hormonal IUD segment held 53.8% share in 2024, supported by its effectiveness in preventing pregnancy and improving menstrual health. Hormonal IUDs, particularly those releasing levonorgestrel, are widely adopted for their long-lasting protection, low maintenance, and added therapeutic advantages, such as reduced menstrual bleeding and relief from endometriosis symptoms. Healthcare providers frequently recommend them due to their dual benefits, strengthening their dominance in the market.
The hospitals segment held 53.2% share in 2024 and is forecasted to generate USD 5.8 billion by 2034. Hospitals remain vital in delivering long-acting reversible contraception, as they provide access to skilled gynecologists and obstetricians for safe insertion procedures. The adoption of postpartum IUD insertion protocols in several regions has broadened access and contributed to a decline in unplanned pregnancies, particularly in areas with robust maternal healthcare systems.
U.S. Intrauterine Devices Market was valued at USD 1.44 billion in 2024 and is estimated to grow at a CAGR of 10.3% between 2025 and 2034. Increased public health initiatives and awareness campaigns have encouraged the use of IUDs as a safe, effective, and convenient contraceptive option. Growing preference for long-acting reversible contraception over short-term alternatives, such as daily pills, has further accelerated adoption across diverse demographics in the U.S. and Canada.
Prominent companies active in the Intrauterine Devices Industry include MONA LISA, Meril, Sebela Pharmaceuticals, DKT, PREGNA, SMB, AbbVie, Prosan, GIMA, Bayer, Medicines360, eurogine, GYNO CARE, CooperSurgical, HLL Lifecare Limited, and others. To strengthen their foothold in the intrauterine devices market, leading companies are focusing on expanding product portfolios with both hormonal and non-hormonal options, including the development of innovative materials designed to minimize side effects and improve user comfort. Strategic collaborations with healthcare organizations and government programs are being pursued to broaden access and adoption rates. Many players are also investing in awareness campaigns to educate women on the benefits of IUDs beyond contraception, such as menstrual health management.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product trends
- 2.2.3 Age group trends
- 2.2.4 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Favorable regulatory scenario
- 3.2.1.2 Rising awareness among women regarding various IUD applications
- 3.2.1.3 High number of unintended pregnancies
- 3.2.1.4 Government initiatives for the prevention of unwanted abortions and pregnancies
- 3.2.1.5 Growing inclination towards planned delayed pregnancy
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of the device
- 3.2.2.2 Risk of several health issues
- 3.2.2.3 Variability in insurance coverage and access
- 3.2.3 Market opportunities
- 3.2.3.1 Rising demand for long-term contraception
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.5.1 Current technologies
- 3.5.2 Emerging technologies
- 3.6 Future market trends
- 3.7 Reimbursement scenario
- 3.8 Consumer behaviour and trends
- 3.9 Brand analysis
- 3.10 Pipeline analysis
- 3.11 Therapeutic applications beyond contraception
- 3.12 Pricing analysis, 2024
- 3.13 Porter's analysis
- 3.14 PESTEL analysis
- 3.15 Gap analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Copper IUD
- 5.3 Hormonal IUD
Chapter 6 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 15-19
- 6.3 20-24
- 6.4 25-29
- 6.5 30-34
- 6.6 35-39
- 6.7 40-44
- 6.8 45+
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Gynecology clinics
- 7.4 Community health care centers
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 AbbVie
- 9.2 Bayer
- 9.3 CooperSurgical
- 9.4 DKT
- 9.5 eurogine
- 9.6 GIMA
- 9.7 GYNO CARE
- 9.8 HLL Lifecare Limited
- 9.9 Medicines360
- 9.10 Meril
- 9.11 MONA LISA
- 9.12 PREGNA
- 9.13 Prosan
- 9.14 Sebela Pharmaceuticals
- 9.15 SMB



















