
|
시장보고서
상품코드
1665063
종양학 기반 In-Vivo CRO 시장 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)Oncology Based In-vivo CRO Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
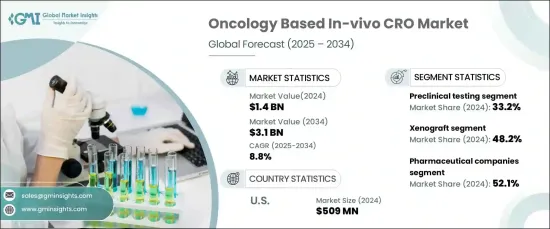
세계의 종양학 기반 In-Vivo CRO 세계 시장은 2024년 14억 달러에 이르렀으며, 2025년부터 2034년까지 연평균 복합 성장률(CAGR) 8.8%의 견조한 성장이 전망되고 있습니다.
이러한 대폭적인 시장 확대의 배경으로는 면역종양학 치료에 대한 수요 증가, 생체내 모델의 지속적인 발전, 생명공학 및 제약 기업 모두에 의한 종양학 치료제의 승인 증가가 있습니다.
소규모 바이오테크놀러지 기업과 제약 기업은 암 치료제 시장 개척을 추진하는 데 중요한 역할을 하고 있어 시장 전체의 성장을 가속시키고 있습니다. 이러한 기업들은 암 영역에서의 암멧 메디컬 니즈에 대응하기 위해 유전자 치료제나 항체 의약을 포함한 혁신적인 치료에 주력하고 있습니다. 이러한 기업의 암 연구에 대한 전문적인 접근법은 전임상시험 및 in-vivo 시험 서비스에 대한 수요를 지속적으로 높여 시장의 상승 궤도에 더욱 기여하고 있습니다.
| 시장 규모 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 14억 달러 |
| 예측 금액 | 31억 달러 |
| CAGR | 8.8% |
종양학 in-vivo CRO 시장은 서비스 유형별로 구분되며, 주요 카테고리는 전임상시험, 효능시험, 독성시험, 약동학 등입니다. 전임상시험은 2024년에 33.2%를 차지했으며 시장에서 가장 큰 점유율을 차지했습니다. 이 이점은 전임상시험이 의약품 개발에서 수행하는 중요한 역할에 기인하며, 인간 임상시험을 진행하기 전에 새로운 치료법의 안전성과 효능을 보장합니다. 미국 FDA 및 EMA와 같은 규제 당국은 임상시험을 시작하기 전에 중요한 단계로 전임상시험을 요구하고 있습니다. 이러한 연구는 약물의 독성, 약력학, 치료 효과에 관한 중요한 데이터를 제공하며, 암 연구 파이프라인에 있어서 필수적인 것이 되고 있습니다.
시장은 또한 이종 이식, 동종 이식 및 기타 모델과 같은 모델 유형에 따라 구분됩니다. 2024년에는 이종이식 모델이 시장을 선도해 48.2%의 점유율을 차지했습니다. 이 모델은 인간 종양 생물학을 충실히 모방할 수 있기 때문에 널리 선호되며 항암제의 효능에 대한 중요한 지식을 제공합니다. 이종이식 모델은 면역 결핍 마우스에 인간 종양 조직을 이식하는 것으로, 인간의 암의 진행을 정확하게 시뮬레이션할 수 있어 약제의 효능의 평가를 높이고 있습니다.
미국에서는 종양학 in-vivo CRO 시장이 2024년 5억 900만 달러를 차지했고 지역별로 우위를 차지했습니다. 이 나라가 시장을 선도하고 있는 것은 암 연구에의 다액의 투자와 제약·바이오테크놀러지 부문의 강도에 기인하고 있습니다. 미국에서는 암치료제 개발에 주력하는 기업이 많이 존재하기 때문에 전임상시험이나 in-vivo 시험을 CRO에 위탁하는 경향이 강합니다. 이 증가 추세는 암 치료 혁신과 생체 내 시험 솔루션에 대한 수요 증가에 힘입어 지속적인 시장 확대를 지원합니다.
목차
제1장 조사 방법과 조사 범위
- 시장 범위와 정의
- 조사 디자인
- 조사 접근
- 데이터 수집 방법
- 기본 추정과 계산
- 기준연도의 산출
- 시장추계의 주요 동향
- 예측 모델
- 1차 조사와 검증
- 1차 정보
- 데이터 마이닝 소스
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 면역종양 치료에 대한 수요 증가
- in-vivo 모델의 진보
- 정밀 종양학에 대한 투자 확대
- 소규모 바이오테크놀러지 기업이나 제약 기업에 의한 암 치료제의 승인 확대
- 업계의 잠재적 위험 및 과제
- 암 치료제의 승인에 대한 엄격한 규제 요건
- 강력한 대체품의 존재
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술적 전망
- 특허 분석
- 장래 시장 동향
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업 점유율 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 전망
제5장 시장 추계·예측 : 서비스별(2021-2034년), 100만 달러
- 주요 동향
- 전임상 시험
- 유효성 시험
- 독성학 연구
- 약동학
- 기타 서비스
제6장 시장 추계·예측 : 모델별(2021-2034년), 100만 달러
- 주요 동향
- 이종 이식
- 환자 유래 이종 이식
- 세포주 유래 이종 이식
- 기타 이종 이식
- 신제네릭
- 기타 모델
제7장 시장 추계·예측 : 최종 용도별(2021-2034년), 100만 달러
- 주요 동향
- 제약기업
- 바이오테크놀러지 기업
- 기타 최종 사용자
제8장 시장 추계·예측 : 지역별(2021-2034년), 100만 달러
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제9장 기업 프로파일
- Charles River Laboratories
- Crown Bioscience
- Eurofins Scientific
- Evotec SE
- ICON plc
- IMV
- Laboratory Corporation of America Holdings
- Medidata
- Merck KGaA
- OncoOne
- Pharmaron
- Taconic Biosciences
- The Jackson Laboratory
- Thermo Fisher Scientific
- WuXi AppTec
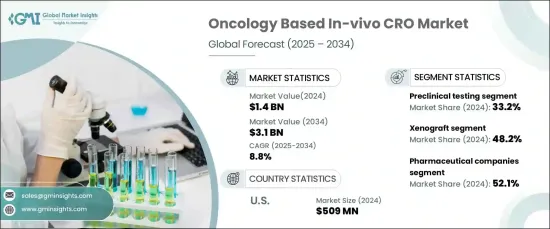
The Global Oncology Based In-Vivo CRO Market reached USD 1.4 billion in 2024 and is expected to grow at a robust compound annual growth rate (CAGR) of 8.8% from 2025 to 2034. This significant market expansion is being driven by the increasing demand for immuno-oncology therapies, ongoing advancements in in-vivo models, and a rise in oncology drug approvals from both biotechnology and pharmaceutical companies.
Small biotechnology and pharmaceutical companies are playing a crucial role in driving the development of oncology drugs, thus accelerating overall market growth. These companies focus on innovative treatments, including gene therapies and antibody-based drugs, which are designed to address unmet medical needs in the oncology space. Their specialized approach to cancer research continues to fuel the demand for preclinical and in-vivo testing services, further contributing to the market's upward trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.4 Billion |
| Forecast Value | $3.1 Billion |
| CAGR | 8.8% |
The oncology in-vivo CRO market is segmented by service type, with the primary categories being preclinical testing, efficacy testing, toxicology studies, pharmacokinetics, and others. Preclinical testing held the largest share of the market in 2024, accounting for 33.2%. This dominance is due to the vital role preclinical testing plays in drug development, ensuring the safety and efficacy of new therapies before they progress to human clinical trials. Regulatory authorities such as the U.S. FDA and EMA require preclinical testing as a critical step before clinical trials can commence. These studies provide essential data on drug toxicity, pharmacodynamics, and therapeutic effects, making them indispensable in the oncology research pipeline.
The market is also segmented by model type, including xenograft, syngeneic, and other models. Xenograft models led the market in 2024, holding a 48.2% share. These models are widely preferred due to their ability to closely mimic human tumor biology, offering crucial insights into the effectiveness of cancer drugs. Xenograft models involve implanting human tumor tissues into immunocompromised mice, which enables an accurate simulation of human cancer progression and enhances the evaluation of drug efficacy.
In the United States, the oncology in-vivo CRO market accounted for USD 509 million in 2024, dominating the regional landscape. The country's market leadership can be attributed to significant investments in oncology research and the strength of its pharmaceutical and biotechnology sectors. With numerous companies focused on oncology drug development, the U.S. heavily relies on outsourcing preclinical and in-vivo studies to CROs. This growing trend supports continued market expansion, driven by innovations in oncology therapies and a rising demand for in-vivo testing solutions.
Table of Contents
Chapter 1 Methodology & Scope
- 1.1 Market scope & definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates & calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing demand for immuno-oncology therapies
- 3.2.1.2 Advancements in in-vivo models
- 3.2.1.3 Growing investments in precision oncology
- 3.2.1.4 Growing oncology drug approvals from small biotechnology and pharmaceutical companies
- 3.2.2 Industry pitfalls & challenges
- 3.2.2.1 Stringent regulatory requirements for oncology drug approvals
- 3.2.2.2 Presence of strong alternatives
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Patent Analysis
- 3.7 Future market trends
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Service, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Preclinical testing
- 5.3 Efficacy testing
- 5.4 Toxicology studies
- 5.5 Pharmacokinetics
- 5.6 Other services
Chapter 6 Market Estimates and Forecast, By Model, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Xenograft
- 6.2.1 Patient derived xenograft
- 6.2.2 Cell-line derived xenograft
- 6.2.3 Other xenografts
- 6.3 Syngeneic
- 6.4 Other models
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical companies
- 7.3 Biotechnology companies
- 7.4 Other end users
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Charles River Laboratories
- 9.2 Crown Bioscience
- 9.3 Eurofins Scientific
- 9.4 Evotec SE
- 9.5 ICON plc
- 9.6 IMV
- 9.7 Laboratory Corporation of America Holdings
- 9.8 Medidata
- 9.9 Merck KGaA
- 9.10 OncoOne
- 9.11 Pharmaron
- 9.12 Taconic Biosciences
- 9.13 The Jackson Laboratory
- 9.14 Thermo Fisher Scientific
- 9.15 WuXi AppTec


















