
|
시장보고서
상품코드
1684642
임상시험 바이오뱅킹 및 아카이브 솔루션 시장 : 기회, 성장 촉진요인, 산업 동향 분석(2025-2034년)Clinical Trial Biorepository and Archiving Solutions Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
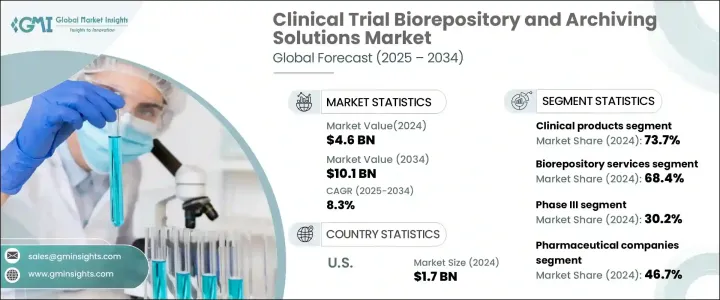
세계의 임상시험 바이오뱅킹 및 아카이브 솔루션 시장은 2024년에는 46억 달러로 평가되었으며, 2025년부터 2034년에 걸쳐 8.3%의 연평균 성장률(CAGR)로 성장할 것으로 예측됩니다.
이러한 성장은 임상시험의 급증, 엄격한 규제 준수 요건, 장기 데이터 저장에 대한 수요 증가, 고급 바이오뱅킹 인프라에 대한 투자 증가 등 몇 가지 중요한 요인에 의해 촉진되고 있습니다. 신약 개발과 개인 맞춤 의학의 획기적인 발전으로 바이오 제약 산업이 계속 발전함에 따라 중요한 생물학적 샘플을 보호하는 바이오 보관소의 역할이 필수 불가결해졌습니다. 이러한 추세는 글로벌 임상시험의 대량 수요를 충족할 수 있는 혁신적인 솔루션에 대한 필요성이 확대되고 있음을 강조합니다.

만성 질환의 유병률 증가와 첨단 치료법에 대한 수요로 인해 제약 및 바이오 제약 회사는 연구 개발 노력을 가속화하고 있습니다. 이러한 기업들은 임상시험의 급증에 앞장서고 있을 뿐만 아니라 최첨단 바이오뱅킹 및 아카이브 시스템에도 막대한 투자를 하고 있습니다. 이러한 시스템은 새로운 치료법의 엄격한 테스트에 필요한 생물학적 샘플의 품질, 안전성, 무결성을 유지하는 데 매우 중요합니다. 또한 자동화된 보관 시스템과 고급 추적 기능과 같은 바이오 보관 솔루션의 기술 발전은 시장의 매력을 더욱 높이고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 46억 달러 |
| 예측 금액 | 101억 달러 |
| CAGR | 8.3% |
시장은 제품 유형별로 전임상 및 임상 제품으로 분류되며, 2024년에는 임상 제품 부문이 73.7%의 시장 점유율로 가장 큰 비중을 차지할 것으로 예상됩니다. 이 부문에는 신약의 효능과 안전성을 테스트하는 데 필수적인 인체 조직, 장기, 줄기세포 및 기타 생물학적 물질이 포함됩니다. 임상시험의 복잡성이 증가하고 이러한 생물학적 샘플을 보존해야 할 필요성이 커지면서 견고한 보관 및 관리 솔루션의 중요성이 강조되고 있습니다.
바이오뱅킹 및 아카이브 솔루션의 최종 사용자에는 제약 회사, 생명공학 회사, 임상시험수탁기관(CRO), 학술 및 연구 기관이 포함됩니다. 2024년에는 제약 회사가 46.7%로 가장 큰 시장 점유율을 차지했는데, 이는 임상시험의 주요 의뢰자로서의 중추적인 역할을 반영하는 결과입니다. 이러한 기업들은 대규모 임상시험에서 수집한 방대한 양의 생물학적 샘플을 관리하기 위해 첨단 바이오뱅킹 서비스에 의존하고 있습니다. 성공적인 임상시험 결과를 달성하기 위해서는 샘플 품질과 규제 표준 준수가 매우 중요합니다.
미국 시장은 2024년에 17억 달러 규모의 시장을 창출했으며, 미국은 계속해서 글로벌 임상 연구를 주도하고 있습니다. 미국에서 실시되는 임상시험의 양이 증가함에 따라 정교한 바이오뱅킹 및 아카이빙 솔루션에 대한 수요가 급증하고 있습니다. 미국의 강력한 연구 인프라와 혁신적인 바이오뱅킹 기술에 대한 막대한 투자는 시장 성장의 핵심 동력으로서 미국의 입지를 공고히 하고 있습니다.
목차
제1장 조사 방법과 조사 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 임상 시험 증가
- 엄격한 규정 준수에 대한 수요 증가
- 장기 데이터 저장 및 보존에 대한 수요 증가
- 바이오뱅킹 인프라 투자 증가
- 업계의 잠재적 위험 및 과제
- 데이터 보안 및 안전과 관련된 우려 사항
- 고급 저장 시설의 자본 집약적 특성
- 성장 촉진요인
- 성장 가능성 분석
- 갭 분석
- 규제 상황
- 기술적 전망
- 장래 시장 동향
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업 점유율 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 전망
제5장 시장 추계 및 예측 : 제품 유형별(2021-2034년)
- 주요 동향
- 전임상 제품
- 임상 제품
- 인테 조직
- 장기
- 줄기세포
- 기타 임상 제품
제6장 시장 추계 및 예측 : 서비스별(2021-2034년)
- 주요 동향
- 바이오뱅킹 서비스
- 창고 보관
- 샘플 처리
- 운송
- 기타 서비스
- 아카이브 솔루션 서비스
- 데이터베이스 인덱싱 및 관리
- 스캔 및 파기
제7장 시장 추계 및 예측 : 페이즈별(2021-2034년)
- 주요 동향
- 전임상
- 1상
- 2상
- 3상
- 4상
제8장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 제약 기업
- 생명 공학 기업
- 수탁 연구 기관
- 학술 연구 기관
제9장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- American Type Culture Collection
- Azenta US Inc.
- BioStorage Technologies
- Celerion
- Celerion
- Charles River Laboratories
- CryoPort
- Hamilton Company
- IQVIA
- Labcorp
- NMDP BioTherapies
- STC Biologics
- Thermo Fisher Scientific
- Veristat
- VWR International(Avantor)
The Global Clinical Trial Biorepository And Archiving Solutions Market was valued at USD 4.6 billion in 2024 and is projected to grow at a robust CAGR of 8.3% from 2025 to 2034. This growth is fueled by several critical factors, including the surging number of clinical trials, stringent regulatory compliance requirements, rising demand for long-term data storage, and increased investments in advanced biobanking infrastructure. As the biopharmaceutical sector continues to evolve with groundbreaking developments in drug discovery and personalized medicine, the role of biorepositories in safeguarding critical biological samples has become indispensable. This trend underscores the expanding need for innovative solutions that can cater to the high-volume demands of global clinical trials.
The increasing prevalence of chronic diseases and the demand for cutting-edge therapies drive pharmaceutical and biopharmaceutical companies to accelerate their research and development efforts. These companies are not only spearheading the surge in clinical trials but also investing heavily in state-of-the-art biobanking and archiving systems. Such systems are crucial for maintaining the quality, safety, and integrity of biological samples required for rigorous testing of novel treatments. Moreover, technological advancements in biorepository solutions, such as automated storage systems and advanced tracking capabilities, further boost the market's appeal.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.6 Billion |
| Forecast Value | $10.1 Billion |
| CAGR | 8.3% |
The market is segmented by product type into preclinical and clinical products, with the clinical products segment dominating at 73.7% of the market share in 2024. This segment encompasses human tissues, organs, stem cells, and other biological materials that are vital for testing the efficacy and safety of new drugs. The growing complexity of clinical trials and the need to preserve these biological samples underscore the importance of robust storage and management solutions.
End users of biorepository and archiving solutions include pharmaceutical companies, biotechnology firms, contract research organizations (CROs), and academic and research institutions. In 2024, pharmaceutical companies held the largest market share at 46.7%, reflecting their pivotal role as primary sponsors of clinical trials. These companies rely on advanced biorepository services to manage the vast volumes of biological samples collected during large-scale clinical studies. Ensuring sample quality and compliance with regulatory standards is critical to achieving successful trial outcomes.
The U.S. market generated USD 1.7 billion in 2024, with the country continuing to lead global clinical research efforts. The growing volume of clinical trials conducted in the U.S. has created an unparalleled demand for sophisticated biorepository and archiving solutions. The nation's strong research infrastructure, coupled with significant investments in innovative biobanking technologies, solidifies its position as a key driver of market growth.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rise in clinical trials
- 3.2.1.2 Growing demand for adhering to stringent regulations
- 3.2.1.3 Increasing demand for long term data storage and preservation
- 3.2.1.4 Increasing investments in biobanking infrastructure
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Concerns related to data security and safety
- 3.2.2.2 Capital intensive nature of advanced storage facilities
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Gap analysis
- 3.5 Regulatory landscape
- 3.6 Technological landscape
- 3.7 Future market trends
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Preclinical products
- 5.3 Clinical products
- 5.3.1 Human tissue
- 5.3.2 Organs
- 5.3.3 Stem cells
- 5.3.4 Other clinical products
Chapter 6 Market Estimates and Forecast, By Services, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Biorepository services
- 6.2.1 Warehousing and storage
- 6.2.2 Sample processing
- 6.2.3 Transportation
- 6.2.4 Other services
- 6.3 Archiving solution services
- 6.3.1 Database indexing and management
- 6.3.2 Scanning and destruction
Chapter 7 Market Estimates and Forecast, By Phase, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Preclinical
- 7.3 Phase I
- 7.4 Phase II
- 7.5 Phase III
- 7.6 Phase IV
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pharmaceutical companies
- 8.3 Biotechnology companies
- 8.4 Contract research organizations
- 8.5 Academic and research institutions
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 American Type Culture Collection
- 10.2 Azenta US Inc.
- 10.3 BioStorage Technologies
- 10.4 Celerion
- 10.5 Celerion
- 10.6 Charles River Laboratories
- 10.7 CryoPort
- 10.8 Hamilton Company
- 10.9 IQVIA
- 10.10 Labcorp
- 10.11 NMDP BioTherapies
- 10.12 STC Biologics
- 10.13 Thermo Fisher Scientific
- 10.14 Veristat
- 10.15 VWR International (Avantor)



















