
|
시장보고서
상품코드
1684650
CAR(키메라 항원 수용체) T세포 치료 시장 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)CAR T-cell Therapy Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
세계의 CAR T세포 치료 시장은 2024년에 43억 달러로 평가되었으며, 2025년부터 2034년까지의 CAGR은 30.5%를 나타낼 전망입니다. 키메라 항원 수용체(CAR) T세포 치료는 암 치료에 혁명을 일으키며 종양학의 중요한 이정표가 되고 있습니다. 이 첨단 형태의 면역 요법은 암세포를 더 잘 표적화하고 제거하기 위해 환자의 T세포를 유전적으로 변형하는 것을 포함합니다. CAR T세포 치료는 환자에게 새로운 희망을 제공할 뿐만 아니라 특정 암의 치료 접근 방식에도 변화를 가져오고 있습니다.
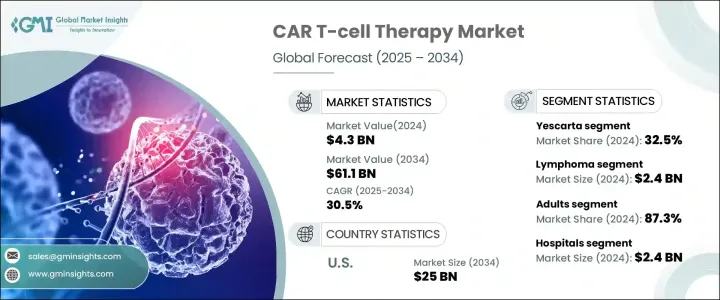
세계 시장은 Abecma, Breyanzi, Carvykti, Kymriah, Tecartus, Yescarta와 같은 주요 제품으로 분류됩니다.특히 기존 치료법이 종종 실패하는 재발성 또는 불응성 B세포 림프종 환자에 대한 효과가 입증되었기 때문입니다. 공격적인 형태의 암에 대한 대안을 찾는 환자가 증가함에 따라 Yescarta에 대한 수요는 계속 증가하고 있으며, Yescarta는 이 분야에서 리더십을 더욱 공고히 하고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 43억 달러 |
| 예측 금액 | 611억 달러 |
| CAGR | 30.5% |
적응증별로 CAR T세포 치료 시장은 백혈병, 림프종, 다발성 골수종 등으로 분류되며, 2024년에는 림프종이 24억 달러의 최고 수익을 올렸습니다. 비호지킨 림프종의 일반적인 형태인 미만성 거대 B세포 림프종(DLBCL)이 이러한 성장의 주요 원인입니다. DLBCL은 기존 치료 후 재발률이 높기 때문에 공격적인 림프종을 표적으로 삼아 치료하는 데 탁월한 효능을 보이는 CAR T세포 치료제에 대한 수요가 증가했습니다. 그 결과, CAR T세포 치료는 이러한 까다로운 암 유형을 관리하는 데 중요한 솔루션으로 점점 더 많이 인식되고 있으며, 남은 옵션이 거의 없는 환자들에게 새로운 희망을 제공하고 있습니다.
미국의 CAR T세포 치료 시장은 2034년까지 250억 달러에 달할 것으로 예상되고 있으며, 규제 프레임워크의 지원에 힘입어 강력한 성장세를 보일 것으로 전망됩니다. 미국 식품의약국(FDA)은 획기적 치료제 지정, 희귀의약품 지정, 패스트트랙 경로와 같은 신속한 승인 절차를 통해 중요한 규제 지원을 제공함으로써 CAR T세포 치료제의 신속한 개발과 상용화에 핵심적인 역할을 해왔습니다.
목차
제1장 조사 방법과 조사 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 인구 전체에서 악성 종양 증가
- 표적 치료에 대한 성향 증가
- 지역별로 승인된 견고한 제품 파이프라인
- 업계의 잠재적 위험 및 과제
- 높은 약품 비용이 시장 성장을 방해
- CAR T세포 치료의 부작용
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술적 전망
- 향후 시장 동향
- 갭 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추계 및 예측 : 제품별(2021-2034년)
- 주요 동향
- Abecma(idecabtagene vicleucel)
- Breyanzi(lisocabtagene maraleucel)
- Carvykti(ciltacabtagene autoleucel)
- Kymriah(tisagenlecleucel)
- Tecartus(brexucabtagene autoleucel)
- Yescarta(axicabtagene ciloleucel)
- 기타 제품
제6장 시장 추계 및 예측 : 적응증별(2021-2034년)
- 주요 동향
- 백혈병
- 림프종
- 다발성 골수종
- 기타 적응증
제7장 시장 추계 및 예측 : 인구 동태별(2021-2034년)
- 주요 동향
- 성인
- 소아
제8장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원
- 암 치료 센터
- 전문 클리닉
제9장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Allogene Therapeutics
- Autolus Therapeutics
- bluebird bio
- Bristol-Myers Squibb Company
- CRISPR Therapeutics
- Gilead Sciences
- GSK plc.
- ImmunoAct
- Johnson &Johnson
- JW Therapeutics(상하이)
- Medigene AG
- Merck KGaA
- Novartis AG
- Sangamo Therapeutics
- Sorrento Therapeutics
The Global CAR T-Cell Therapy Market, valued at USD 4.3 billion in 2024, is projected to experience a robust growth trajectory, with an impressive CAGR of 30.5% from 2025 to 2034. Chimeric Antigen Receptor (CAR) T-cell therapy is revolutionizing cancer treatment, marking a significant milestone in oncology. This advanced form of immunotherapy involves the genetic modification of a patient's T-cells to better target and eliminate cancer cells. CAR T-cell therapy is not only offering new hope for patients but also changing the way we approach the treatment of certain cancers. With its growing adoption and advancements in technology, this market is poised for continuous expansion.
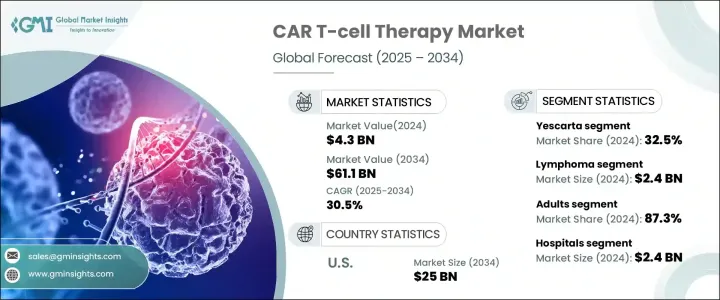
The global market is divided into several key products, including Abecma, Breyanzi, Carvykti, Kymriah, Tecartus, Yescarta, and others. Among these, Yescarta stands out as the market leader, capturing 32.5% of the total market share in 2024. The primary reason for Yescarta's market dominance lies in its proven effectiveness for patients with B-cell lymphomas, particularly in relapsed or refractory cases where traditional treatments often fall short. As more patients seek alternatives for aggressive forms of cancer, the demand for Yescarta continues to grow, further cementing its leadership in the space.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.3 Billion |
| Forecast Value | $61.1 Billion |
| CAGR | 30.5% |
In terms of indications, the CAR T-cell therapy market is categorized into leukemia, lymphoma, multiple myeloma, and others, with lymphoma generating the highest revenue of USD 2.4 billion in 2024. Diffuse large B-cell lymphoma (DLBCL), a common form of non-Hodgkin lymphoma is a major contributor to this growth. DLBCL's high relapse rates following conventional therapies have driven demand for CAR T-cell treatments, which have shown remarkable efficacy in targeting and treating aggressive lymphoma. As a result, CAR T-cell therapies are increasingly viewed as a vital solution in managing these challenging cancer types, offering new hope to patients with few options left.
The U.S. CAR T-cell therapy market is expected to reach USD 25 billion by 2034, with strong growth fueled by supportive regulatory frameworks. The U.S. Food and Drug Administration (FDA) has played a key role in the rapid development and commercialization of CAR T-cell therapies, providing critical regulatory support through expedited approval processes such as Breakthrough Therapy Designations, Orphan Drug status, and Fast Track pathways. These initiatives have fast-tracked the availability of CAR T-cell therapies, ensuring timely access for patients in need of life-saving treatments.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing cases of cancer malignancies across population
- 3.2.1.2 Rising inclination towards targeted treatment
- 3.2.1.3 Robust product pipeline with regulatory approvals across geographies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High drug cost impedes the market growth
- 3.2.2.2 Side-effects of CAR T-cell therapy
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Abecma (idecabtagene vicleucel)
- 5.3 Breyanzi (lisocabtagene maraleucel)
- 5.4 Carvykti (ciltacabtagene autoleucel)
- 5.5 Kymriah (tisagenlecleucel)
- 5.6 Tecartus (brexucabtagene autoleucel)
- 5.7 Yescarta (axicabtagene ciloleucel)
- 5.8 Other products
Chapter 6 Market Estimates and Forecast, By Indication, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Leukemia
- 6.3 Lymphoma
- 6.4 Multiple myeloma
- 6.5 Other indications
Chapter 7 Market Estimates and Forecast, By Demographic, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Adults
- 7.3 Pediatric
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Cancer treatment centers
- 8.4 Specialty clinics
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Allogene Therapeutics
- 10.2 Autolus Therapeutics
- 10.3 bluebird bio
- 10.4 Bristol-Myers Squibb Company
- 10.5 CRISPR Therapeutics
- 10.6 Gilead Sciences
- 10.7 GSK plc.
- 10.8 ImmunoAct
- 10.9 Johnson & Johnson
- 10.10 JW Therapeutics (Shanghai)
- 10.11 Medigene AG
- 10.12 Merck KGaA
- 10.13 Novartis AG
- 10.14 Sangamo Therapeutics
- 10.15 Sorrento Therapeutics



















