
|
시장보고서
상품코드
1699266
인플루엔자 신속 진단 검사(RIDT) 시장 : 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Rapid Influenza Diagnostic Tests (RIDT) Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034 |
||||||
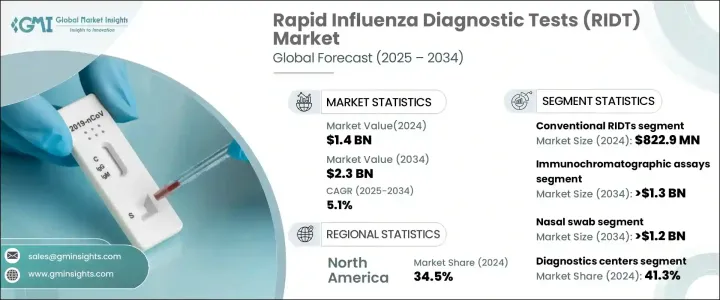
세계의 인플루엔자 신속 진단 검사 시장은 2024년에 14억 달러에 이르렀으며, 2025-2034년 연평균 복합 성장률(CAGR) 5.1%로 성장할 것으로 예측됩니다.
계절성 인플루엔자의 유행 증가, 포인트 오브 케어 검사의 진보, 진단 방법의 지속적인 혁신이 시장의 성장을 가속하고 있습니다. 세계 각국의 정부는 인플루엔자 모니터링을 강화하고 계발 캠페인을 실시하고 있으며, RIDT의 채용을 더욱 확대하고 있습니다. 기술의 진보는 검사 정확도를 크게 향상시키며, 디지털 RIDT는 감도와 특이도를 높이고 있습니다.

정성적 RIDT에서 반정량적 RIDT로의 전환은 임상적 타당성을 높여 여러 호흡기 병원체를 검출할 수 있는 다중화 검사는 일반적으로 되고 있습니다. 인공지능(AI) 및 머신러닝은 검사 해석을 최적화해 오류를 줄이고 진단을 보다 친숙하게 만들고 있습니다. 헬스케어 인프라에 대한 투자의 확대도, 이러한 검사의 가용성을 향상시키고 있습니다. 규제 기관은 합리화된 승인 및 공중 보건 이니셔티브에 대한 재정 지원을 통해 인플루엔자 신속 진단 검사를 지원하고 있습니다. 진단 검사에 대한 자금 지원이 증가함으로써 일반 시민의 의식이 높아지고 조기 발견 및 조기 치료가 촉진되어 시장이 전진하고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 14억 달러 |
| 예측 금액 | 23억 달러 |
| CAGR | 5.1% |
RIDTs 시장은 제품 유형, 기술, 샘플 유형, 최종 용도 및 지역별로 구분됩니다. 전통적인 RIDTs 부문은 2024년에 8억 2,290만 달러를 창출했습니다. 이러한 검사는 저렴한 가격으로 구하기 쉽기 때문에 특히 헬스케어 인프라가 제한된 지역에서 널리 사용되고 있습니다. 심플한 디자인으로 제조 비용이 낮아 공중 위생 프로그램에서 사용하기에 이상적이며, 폭넓은 이용이 가능합니다. 사용하기 쉽고 필요한 훈련도 최소한이기 때문에 진료소, 직장, 학교 등 다양한 환경에서 실시할 수 있습니다. 신속한 결과는 신속한 의사결정을 가능하게 하여 계절 인플루엔자 유행 관리에 매우 효과적입니다.
기술별로, 면역 크로마토 분석은 CAGR 5.9%의 예측으로 시장 성장을 이끌고 2034년까지 13억 달러 이상에 이를 것으로 예상되고 있습니다. 이러한 검사는 민감성과 특이성이 높아 소아나 노인과 같은 취약한 집단에서 독감 검출에 특히 유용합니다. 면역 크로마토 분석의 수요는 약국이나 응급실 등 분산된 헬스케어 환경에서 증가하고 있습니다. 면역 크로마토 분석은 콤팩트한 설계로 필요한 장비도 최소한이기 때문에 이러한 장소에서 사용하기에 적합합니다. 지속적인 기술의 진보로 속도 및 정확도가 더욱 향상되어 헬스케어 전문가들 사이에서 선호되는 선택지가 되고 있습니다.
샘플 유형별로 비강 면봉 분야는 CAGR 5.9%로 성장하여 2034년까지 12억 달러 이상에 달할 것으로 예측되고 있습니다. 비강 면봉은 적용이 간단하고 독감 검출 정확도가 높기 때문에 널리 사용되고 있습니다. 다른 샘플 채취법보다 침습성이 낮기 때문에 소아에서 성인까지 적합합니다. 높은 바이러스 양을 잡을 수 있기 때문에 인플루엔자 조기 진단에 효과적이며 포인트 오브 케어 및 랩 검사 모두의 환경에서 헬스케어 공급자에게 이익을 가져다 주고 있습니다.
진단센터는 2024년에 41.3%로 가장 높은 최종 용도의 판매 점유율을 차지했습니다. 이러한 시설은 고도의 진단 기술을 제공하고 훈련받은 전문가를 고용함으로써 정확하고 효율적인 검사를 실현하고 있습니다. 여러 호흡기 감염병에 대한 멀티플렉스 검사 등 종합적인 진단 서비스에 대한 수요 증가가 진단센터의 RIDT 의존도를 높이는 한 요인이 되고 있습니다. 이러한 시설은 대규모 인플루엔자 모니터링 프로그램에서 중요한 역할을 하고 있어 시장의 우위성을 강화하고 있습니다.
북미는 확립된 헬스케어 시스템, 신속검사 키트의 보급, 포인트 오브 케어 검사의 채용 증가로 2024년에는 34.5%의 최대 시장 점유율을 획득했습니다. 미국 시장은 2021년에 3억 3,100만 달러, 2022년에 3억 6,580만 달러, 2023년에 4억 130만 달러로 평가되었으며, 이 지역에서의 우위성을 반영하고 있습니다.
목차
제1장 조사 방법 및 조사 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 인플루엔자의 유행 확대
- 기술의 진보
- 인플루엔자의 조기 진단 및 관리에 대한 수요 증가
- 신속 진단 검사의 보급
- 업계의 잠재적 위험 및 과제
- 숙련된 전문가의 부족
- 엄격한 규제
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술 상황
- 가격 분석
- 갭 분석
- Porter's Five Forces 분석
- PESTEL 분석
- 밸류체인 분석
제4장 경쟁 구도
- 서문
- 기업 매트릭스 분석
- 기업 점유율 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추계 및 예측 : 제품 유형별(2021-2034년)
- 주요 동향
- 기존 RIDT
- 디지털 RIDT
제6장 시장 추계 및 예측 : 기술별(2021-2034년)
- 주요 동향
- 면역 크로마토 분석
- 래터럴 플로우 분석
- PCR법
- 기타 기술
제7장 시장 추계 및 예측 : 샘플 유형별(2021-2034년)
- 주요 동향
- 비강 면봉
- 인두 면봉
- 기타 샘플 유형
제8장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 진단센터
- 병원
- 연구실
- 기타 최종 용도
제9장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- 3B BlackBio
- Abbott
- Access Bio
- BD
- bioMerieux
- CHEMBIO
- DiaSorin
- Meridian
- Quidel Corporation
- Roche
- SEKISUI
- Siemens HEALTHINEERS
- Thermo Fisher
The Global Rapid Influenza Diagnostic Tests Market reached USD 1.4 billion in 2024 and is projected to grow at a CAGR of 5.1% from 2025 to 2034. The rising prevalence of seasonal flu, advancements in point-of-care testing, and continuous innovations in diagnostic methods are driving market growth. Governments worldwide are strengthening influenza surveillance and running awareness campaigns, further expanding the adoption of RIDTs. Technological advancements have significantly improved test accuracy, with digital RIDTs offering enhanced sensitivity and specificity.
The shift from qualitative to semi-quantitative RIDTs has increased their clinical relevance, and multiplexed tests capable of detecting multiple respiratory pathogens are becoming more common. Artificial intelligence (AI) and machine learning are optimizing test interpretation, reducing errors, and making diagnostics more accessible. Growing investments in healthcare infrastructure are also improving the availability of these tests. Regulatory bodies are supporting rapid influenza diagnosis through streamlined approvals and financial support for public health initiatives. Increased funding for diagnostic testing is helping expand public awareness, encouraging early detection and treatment, and driving the market forward.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.4 Billion |
| Forecast Value | $2.3 Billion |
| CAGR | 5.1% |
The RIDTs market is segmented based on product type, technology, sample type, end-use, and region. The conventional RIDTs segment generated USD 822.9 million in 2024. These tests remain widely used due to their affordability and ease of access, particularly in regions with limited healthcare infrastructure. Their simple design and low production cost make them ideal for use in public health programs, ensuring broad availability. The ease of use and minimal training requirements allow these tests to be implemented in a variety of settings, including clinics, workplaces, and schools. Fast results enable quick decision-making, making them highly effective in managing seasonal flu outbreaks.
By technology, immunochromatographic assays are expected to lead market growth with a projected CAGR of 5.9%, reaching over USD 1.3 billion by 2034. These tests offer high sensitivity and specificity, making them particularly valuable for detecting influenza in vulnerable populations, such as children and the elderly. The demand for immunochromatographic assays is increasing in decentralized healthcare settings, including pharmacies and urgent care centers. Their compact design and minimal equipment requirements make them well-suited for such locations. Continued technological advancements have further improved their speed and accuracy, making them a preferred choice among healthcare professionals.
Based on sample type, the nasal swab segment is projected to grow at a CAGR of 5.9%, surpassing USD 1.2 billion by 2034. Nasal swabs are widely used due to their ease of application and high accuracy in detecting influenza. They are less invasive than other sample collection methods, making them suitable for both children and adults. Their ability to capture high viral loads makes them effective in early influenza diagnosis, benefiting healthcare providers in both point-of-care and laboratory testing environments.
Diagnostic centers accounted for the highest end-use revenue share of 41.3% in 2024. These facilities offer advanced diagnostic technologies and employ trained professionals, ensuring accurate and efficient testing. The increasing demand for comprehensive diagnostic services, including multiplex testing for multiple respiratory infections, has contributed to the growing reliance on RIDTs in diagnostic centers. These facilities play a crucial role in large-scale influenza monitoring programs, reinforcing their market dominance.
North America captured the largest market share of 34.5% in 2024, driven by a well-established healthcare system, the widespread availability of rapid test kits, and the increasing adoption of point-of-care testing. The US market was valued at USD 331 million in 2021, rising to USD 365.8 million in 2022 and USD 401.3 million in 2023, reflecting its dominant position within the region.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of influenza
- 3.2.1.2 Technological advancements
- 3.2.1.3 Rising demand for early influenza diagnosis and management
- 3.2.1.4 Increasing popularity of rapid diagnostic tests
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of skilled professionals
- 3.2.2.2 Stringent regulations
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technology landscape
- 3.6 Pricing analysis
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
- 3.10 Value chain analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Conventional RIDTs
- 5.3 Digital RIDTs
Chapter 6 Market Estimates and Forecast, By Technology, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Immunochromatographic assays
- 6.3 Lateral flow assays
- 6.4 PCR
- 6.5 Other technologies
Chapter 7 Market Estimates and Forecast, By Sample Type, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Nasal swab
- 7.3 Throat swab
- 7.4 Other sample types
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Diagnostic centers
- 8.3 Hospitals
- 8.4 Research laboratories
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 3B BlackBio
- 10.2 Abbott
- 10.3 Access Bio
- 10.4 BD
- 10.5 bioMérieux
- 10.6 CHEMBIO
- 10.7 DiaSorin
- 10.8 Meridian
- 10.9 Quidel Corporation
- 10.10 Roche
- 10.11 SEKISUI
- 10.12 Siemens HEALTHINEERS
- 10.13 Thermo Fisher



















