
|
시장보고서
상품코드
1699415
파브리병 치료 시장 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)Fabry Disease Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034 |
||||||
세계 패브리병 치료 시장은 2024년 23억 달러로 평가되었고, 2025-2034년 연평균 8.2% 성장할 것으로 예상됩니다.
파브리병에 대한 매우 효과적이고 표적화된 특이적 치료제에 대한 수요 증가가 세계 시장 확대의 원동력이 되고 있습니다. 파브리병은 GLA 유전자의 돌연변이로 인해 발생하는 희귀 유전성 질환으로 다양한 장기에 유해한 지방 물질이 축적되어 신장, 심장, 신경계에 영향을 미치는 심각한 합병증을 유발하는 희귀 유전질환입니다. 파브리병에 대한 인식이 높아짐에 따라 의료진과 제약사들은 증상과 근본적인 원인을 모두 해결할 수 있는 혁신적인 치료법 개발에 집중하고 있습니다. 지속적인 연구와 임상적 발전으로 새로운 치료 옵션이 생겨나면서 환자의 예후를 개선하고 삶의 질을 향상시킬 수 있는 새로운 치료법이 등장하고 있습니다. 민관 양 부문의 자금 지원 증가와 바이오 제약사간의 제휴로 이 시장에서의 기술 혁신이 가속화될 것으로 예상됩니다. 새로운 치료제의 패스트트랙 지정에 따른 규제 당국의 지원으로 의약품 승인이 더욱 빨라지고 있으며, 이는 업계 참여자들에게 유리한 기회를 창출하고 있습니다. 경쟁 환경은 진화하고 있으며, 기업들은 보다 효과적이고 접근하기 쉬운 치료법을 시장에 출시하기 위해 연구개발에 많은 투자를 하고 있습니다.

시장은 여러 치료법으로 분류되지만, 효소대체요법(ERT)이 선두를 달리고 있으며, 2024년 ERT 시장 규모는 17억 달러로, 파브리병의 표준 치료제로서의 역할이 확립되어 있기 때문에 계속 우위를 점하고 있습니다. 이 치료법은 결핍된 효소인 알파-갈락토시다아제 A를 보충함으로써 효과를 발휘하며, 이 질환의 관리와 진행성 장기 손상을 예방하는 데 필수적인 요소입니다. 파브리병의 유병률 증가는 ERT에 대한 수요를 직접적으로 증가시켰고, 진단 방법의 개선으로 더 많은 환자들이 치료를 받을 수 있게 되었습니다. 차세대 시퀀싱과 효소 분석은 파브리병 진단에 혁명을 일으켜 보다 빠르고 정확한 진단을 가능하게 하여 ERT의 채택을 더욱 촉진하고 있습니다. 반감기가 연장되고 효능이 개선된 차세대 ERT 제제의 지속적인 연구로 이 분야의 성장이 지속될 것으로 예상됩니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 23억 달러 |
| 예상 금액 | 51억 달러 |
| CAGR | 8.2% |
이 시장은 또한 정맥 투여와 경구 투여 등 투여 경로에 따라 치료법을 구분하고 있습니다. 정맥 치료는 2024년 68.3%의 점유율을 차지하며 2025-2034년 CAGR 8.1%로 성장세를 이어갈 것으로 예상됩니다. 파브리병에 대한 인식이 높아지고 유전자 스크리닝 프로그램이 개선되면서 진단 건수가 증가하고 있으며, 이는 정맥주사 치료 수요를 견인하고 있습니다. 임상연구에서 ERT의 정맥 투여가 장기적으로 증상을 안정화시키는 데 효과적이라는 사실이 일관되게 밝혀져 선호되는 치료법으로 자리 잡았습니다. 그러나 경구용 요법은 잠재적인 편의성과 환자의 순응도 향상으로 인해 주목을 받고 있습니다.
북미는 2024년 9억 4,580만 달러의 파브리병 치료제 시장에서 9억 4,800만 달러의 매출을 올리며 시장 확대의 주요 지역으로 부상할 것으로 예상됩니다. 성장의 원동력은 정부의 적극적인 노력, 유병률 증가, 희귀질환 치료를 전문으로 하는 주요 헬스케어 기업의 존재입니다. 미국 내 치료 건수 증가는 높은 의료비 및 강력한 상환 정책과 함께 시장 성장에 크게 기여하고 있습니다. 이 지역은 파브리병 치료의 연구 개발의 최전선에 있으며, 여러 임상시험과 신약 승인으로 경쟁 구도를 형성하고 있습니다.
목차
제1장 조사 방법과 조사 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 대한 영향요인
- 성장 촉진요인
- 업계의 잠재적 리스크&과제
- 성장 가능성 분석
- 향후 시장 동향
- 가격 분석
- 제품 파이프라인 분석
- 규제 상황
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업 매트릭스 분석
- 기업 점유율 분석
- 주요 시장 기업 - 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추산 및 예측 : 치료별, 2021년-2034년
- 주요 동향
- 효소 보충 요법(ERT)
- 샤프롱 치료
- 기타 치료
제6장 시장 추산 및 예측 : 투여 경로별, 2021년-2034년
- 주요 동향
- 정맥내 투여
- 경구
제7장 시장 추산 및 예측 : 최종 용도별, 2021년-2034년
- 주요 동향
- 병원
- 재택치료
- 기타 최종 용도
제8장 시장 추산 및 예측 : 지역별, 2021년-2034년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 인도
- 일본
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카공화국
- 사우디아라비아
- 아랍에미리트(UAE)
제9장 기업 개요
- Amicus Therapeutics
- Avrobio
- Freeline Therapeutics
- Idorsia Pharmaceuticals
- ISU Abxis
- JCR Pharmaceuticals
- Novartis
- Pfizer
- Protalix BioTherapeutics
- Sanofi
- Takeda Pharmaceuticals
- Viatris
The Global Fabry Disease Treatment Market was valued at USD 2.3 billion in 2024 and is projected to grow at a CAGR of 8.2% during 2025-2034. The increasing demand for highly effective, targeted, and specific treatments for Fabry disease is driving market expansion worldwide. The condition, a rare genetic disorder caused by mutations in the GLA gene, leads to the buildup of harmful fatty substances in various organs, resulting in severe complications affecting the kidneys, heart, and nervous system. As awareness of Fabry disease continues to grow, healthcare providers and pharmaceutical companies are focusing on developing innovative therapies that address both the symptoms and underlying causes. With ongoing research and clinical advancements, new treatment options are emerging, giving patients improved outcomes and an enhanced quality of life. Increased funding from both public and private sectors, along with collaborations among biopharmaceutical companies, is expected to accelerate innovation in this market. Regulatory support, along with fast-track designations for novel therapies, is further expediting drug approvals, creating lucrative opportunities for industry participants. The competitive landscape is evolving, with companies investing heavily in research and development to bring more effective and accessible treatments to market.
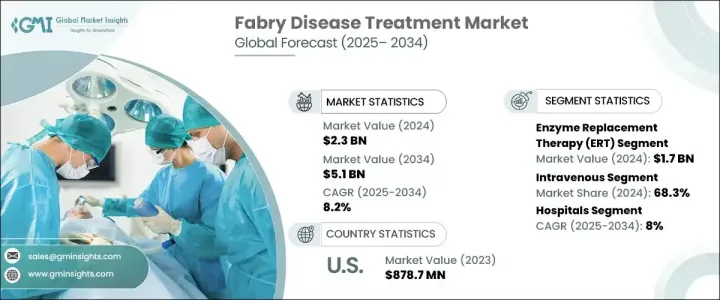
The market is categorized into several treatment options, with enzyme replacement therapy (ERT) leading the way. ERT was valued at USD 1.7 billion in 2024 and continues to dominate due to its established role as the standard treatment for Fabry disease. This therapy works by replacing the deficient enzyme alpha-galactosidase A, an essential component in managing the disease and preventing progressive organ damage. The rising prevalence of Fabry disease has directly increased the demand for ERT, with more patients gaining access to treatment due to improved diagnostic methods. Next-generation sequencing and enzyme assays have revolutionized Fabry disease diagnosis, enabling earlier and more accurate detection, which is further fueling the adoption of ERT. Ongoing research into next-generation ERT formulations with extended half-lives and improved efficacy is expected to sustain growth in this segment.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2.3 Billion |
| Forecast Value | $5.1 Billion |
| CAGR | 8.2% |
The market also distinguishes treatments based on their route of administration, including intravenous and oral options. Intravenous treatments accounted for a 68.3% share in 2024 and are expected to continue growing at a CAGR of 8.1% during 2025-2034. The increasing awareness of Fabry disease and improvements in genetic screening programs have led to more diagnoses, which is driving demand for intravenous therapies. Clinical studies consistently show that intravenous ERT is more effective at stabilizing symptoms over the long term, making it the preferred treatment. However, oral therapies are gaining attention due to their potential convenience and improved patient adherence.
North America generated USD 945.8 million in revenue from the Fabry Disease Treatment Market in 2024, making it a key region for market expansion. Growth is driven by favorable government initiatives, rising incidence rates, and the presence of leading healthcare companies specializing in rare disease treatments. The increasing number of treatment procedures in the U.S., coupled with high healthcare expenditure and strong reimbursement policies, has significantly contributed to market growth. The region remains at the forefront of research and development in Fabry disease treatments, with multiple clinical trials and new drug approvals shaping the competitive landscape.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising cases of fabry disease across the globe
- 3.2.1.2 Growing awareness among specialists and physicians
- 3.2.1.3 Advancements in fabry disease treatment therapies
- 3.2.1.4 Rising health awareness and demand for early-stage diagnosis
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High treatment cost
- 3.2.2.2 Limited treatment options
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Future market trends
- 3.5 Pricing analysis
- 3.6 Product pipeline analysis
- 3.7 Regulatory landscape
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Treatment, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Enzyme replacement therapy (ERT)
- 5.3 Chaperone treatment
- 5.4 Other treatment types
Chapter 6 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Intravenous
- 6.3 Oral
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Homecare settings
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Amicus Therapeutics
- 9.2 Avrobio
- 9.3 Freeline Therapeutics
- 9.4 Idorsia Pharmaceuticals
- 9.5 ISU Abxis
- 9.6 JCR Pharmaceuticals
- 9.7 Novartis
- 9.8 Pfizer
- 9.9 Protalix BioTherapeutics
- 9.10 Sanofi
- 9.11 Takeda Pharmaceuticals
- 9.12 Viatris
















