
|
시장보고서
상품코드
1766182
수두 백신 시장 : 기회, 성장 촉진 요인, 산업 동향 분석, 예측(2025-2034년)Varicella Vaccine Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
세계 수두 백신 시장은 2024년에는 34억 달러에 달했고, CAGR 6.5%로 성장하고, 2034년에는 63억 달러에 이를 것으로 추정됩니다. 수두의 세계 부담 증가는 이 시장의 주요 촉진요인이 되고 있습니다. 감염률이 높고 특히 인구밀도가 높은 지역에서 반복적으로 발생하기 때문에 효과적인 예방접종 솔루션에 대한 수요가 높아지고 있습니다. 조기 예방 접종에 대한 의식이 높아짐에 따라 정부 주도의 강력한 예방 접종 캠페인과 자금 지원과 함께 수두 백신의 세계 보급이 가속화되고 있습니다. 특히 필요한 접종 횟수를 줄이고 환자의 컴플라이언스를 향상시키는 혼합 백신의 개발이 진행되고 있습니다.
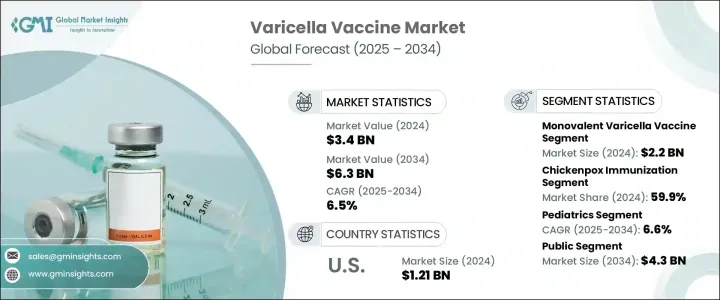
국가 프로그램의 통합 예방 접종 스케줄로의 전환이 진행되고 있는 것도 접종률의 향상에 기여하고 있습니다. 게다가, 헬스케어 제도가 예방 대책과 정기적인 예방 접종을 중시하고 있기 때문에 수요도 증가의 일도를 추적하고 있습니다. 일부 지역에서는 노인이 증가하고 있어 수두 및 대상포진의 재활성화에 걸리기 쉽기 때문에 백신접종에 대한 관심이 지속되고 있는 것도 일인이 되고 있습니다. 헬스케어에 대한 접근이 확대되고, 백신접종의 보급과 저가격화를 목표로 한 관민 파트너십이 진행되고 있기 때문에 수두 백신 시장은 앞으로도 안정된 성장을 이룰 것으로 예측됩니다.
| 시장 규모 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 34억 달러 |
| 예측 금액 | 63억 달러 |
| CAGR | 6.5% |
단가 수두 백신 부문은 2024년 평가 금액이 22억 달러로 시장을 선도했습니다. 일상적인 예방 접종 프로그램, 특히 자원이 제한된 국가에서 널리 사용되는 것이 이러한 지배력을 뒷받침합니다. 이 백신은 다가 대체 백신보다 저렴하고 보관하기 쉽고 로지스틱하게 간단하며 대규모 공중 보건 캠페인에 이상적입니다. 장기간에 걸쳐 일관되게 사용되어 왔으며, 보건 당국과 임상의 사이에서 강한 신뢰가 구축되어 인기 유지에 도움이 되고 있습니다. 세계는 어린 시절의 예방 접종 일정에서 선호되는 옵션입니다.
2024년에는 수두 예방 접종 부문이 59.9%로 가장 큰 점유율을 차지했습니다. 첨단 건강 관리 시스템이있는 국가에서는 수두 예방 접종을 보편적 인 예방 접종 시책에 통합하고 있으며, 결과적으로 높은 섭취율을 얻을 수 있습니다. 에의 접근 강화, 보호자의 의식의 높아, 수두 백신 접종을 추진하는 국가 프로그램이 이 동향을 뒷받침하고 있습니다.이 바이러스는 감염력이 강하기 때문에 각국은 백신에의 액세스와 접종을 계속 우선하고 있습니다.
북미 수두 백신 2024년 시장 점유율은 39.1% 이 성장을 지원하고 있는 것은 확립된 헬스케어 시스템과 적극적인 규제 환경입니다. 이 지역의 공중 보건 기관은 수두 백신이 널리 보급되고 보험 계획에 따라 환급될 수 있도록 지원하여 개인의 직접 비용을 최소화했습니다. 백신의 조기 가용성, 높은 보험 적용률, 강력한 공중 보건 메시지로 인해 상당한 보급이 촉진되었습니다. 그 결과,이 지역은 계속해서 왕성한 수요를 보여 주며, 예측 기간을 통해 세계 수두 백신 시장의 지배적 공헌 요인이 될 것으로 예측됩니다.
수두 백신 세계 시장에서 주요 기업으로는 SK Bioscience, Sanofi, GC Pharma(Green Cross Holdings), GlaxoSmithKline, Takeda Pharmaceutical Company Limite, Sequirus, Mitsubishi Tanabe Pharma Corporation, Novo Medi Sciences, Merck-Sinovac Changsheng Life Sciences Limited 등이 있습니다. 수두 백신 시장의 주요 기업은 시장에서의 존재감을 높이기 위해 다양한 전략적 이니셔티브를 활용하고 있습니다. 수두 예방을 보다 광범위한 예방접종 스케줄에 통합하여 편리성과 컴플라이언스를 향상시키는 혼합 백신을 개발하기 위한 연구개발에도 투자하고 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 산업 고찰
- 생태계 분석
- 공급자의 상황
- 각 단계에서의 부가가치
- 밸류체인에 영향을 주는 요인
- 산업에 미치는 영향요인
- 성장 촉진요인
- 수두의 만연 증가
- 정부에 의한 예방접종 프로그램의 확대
- 백신 개발에 있어서의 기술의 진보
- 산업의 잠재적 리스크 및 과제
- 엄격한 규제 요건
- 백신의 보관 및 운송에 걸리는 고비용
- 시장 기회
- 국가 예방 접종 프로그램의 확대
- 성인과 청소년의 백신접종 수요 증가
- 혼합 백신의 성장
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 기술
- 현재의 기술 동향
- 신흥기술
- 장래 시장 동향
- 파이프라인 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 합병과 인수
- 파트너십 및 협업
- 신제품 발매
- 확대 계획
제5장 시장 추정 및 예측 : 백신 유형별, 2021-2034년
- 주요 동향
- 1가의 수두 백신
- 조합 수두 백신
제6장 시장 추정 및 예측 : 용도별, 2021-2034년
- 주요 동향
- 수두 예방 접종
- 오타후쿠 감기, 홍역, 풍진, 수두(MMRV)의 예방접종
제7장 시장 추정 및 예측 : 연령층별, 2021-2034년
- 주요 동향
- 소아
- 청소년과 성인
제8장 시장 추정 및 예측 : 조달별, 2021-2034년
- 주요 동향
- 공공
- 민간
제9장 시장 추정 및 예측 : 지역별, 2021-2034년
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Bio-Med
- Changchun Changsheng Life Sciences Limited
- GC Pharma(Green Cross Holdings)
- GlaxoSmithKline
- Merck
- Mitsubishi Tanabe Pharma Corporation
- Novo Medi Sciences
- Sanofi
- Seqirus
- Sinovac BIoTech
- SK Bioscience
- Takeda Pharmaceutical Company Limited
The Global Varicella Vaccine Market was valued at USD 3.4 billion in 2024 and is estimated to grow at a CAGR of 6.5% to reach USD 6.3 billion by 2034. The increasing global burden of varicella, or chickenpox, continues to be a major driver of this market. High transmission rates and recurring outbreaks, especially in densely populated regions, are raising the demand for effective immunization solutions. The rising awareness of early vaccination, combined with strong government-led immunization campaigns and funding support, is accelerating the uptake of varicella vaccines globally. Technological advancements are also shaping the market's momentum, particularly the development of combination vaccines that reduce the number of required doses and enhance patient compliance.
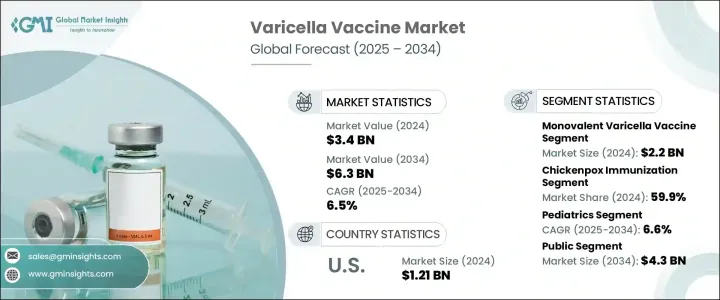
The growing shift toward integrated immunization schedules in national programs is helping increase coverage. Additionally, as healthcare systems emphasize preventive measures and routine immunization, demand continues to grow. The increasing elderly population in several regions has also contributed to sustained interest in vaccination due to susceptibility to varicella-zoster reactivation. With expanded access to healthcare and ongoing public-private partnerships aimed at improving vaccine reach and affordability, the market for varicella vaccines is anticipated to experience consistent and steady growth in the years ahead.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.4 Billion |
| Forecast Value | $6.3 Billion |
| CAGR | 6.5% |
The monovalent varicella vaccine segment led the market with a valuation of USD 2.2 billion in 2024. Its widespread use in routine immunization programs, especially in countries with limited resources, supports this dominance. These vaccines are often more affordable, easier to store, and logistically simpler than multivalent alternatives, making them ideal for large-scale public health campaigns. Their consistent usage over time has built strong trust among health authorities and clinicians, helping maintain their popularity. Proven efficacy and long-standing safety records have made monovalent vaccines the preferred choice for childhood immunization schedules globally. They continue to gain traction in both developed and developing countries due to their reliability and cost-efficiency.
In 2024, the chickenpox immunization segment accounted for the largest share at 59.9%. This leadership is attributed to the widespread demand for childhood varicella protection. Countries with advanced healthcare systems have integrated varicella vaccination into their universal immunization policies, resulting in high uptake. Even in emerging markets, stronger healthcare access, growing parental awareness, and national programs promoting varicella vaccination are helping drive this trend. With the virus being highly contagious, countries continue to prioritize vaccine access and delivery. Continuous education and outreach efforts are increasing compliance and making immunization against chickenpox a standard part of pediatric healthcare globally.
North America Varicella Vaccine Market held a 39.1% share in 2024. This growth is supported by well-established healthcare systems and proactive regulatory environments. Public health agencies in the region have helped ensure that varicella vaccines are widely available and reimbursed under insurance plans, minimizing direct costs for individuals. The early availability of the vaccine, high insurance coverage, and strong public health messaging have driven significant adoption. As a result, the region continues to show strong demand and is expected to remain a dominant contributor to the global varicella vaccine market throughout the forecast period.
Leading companies in the Global Varicella Vaccine Market include SK Bioscience, Sanofi, GC Pharma (Green Cross Holdings), GlaxoSmithKline, Takeda Pharmaceutical Company Limited, Seqirus, Mitsubishi Tanabe Pharma Corporation, Novo Medi Sciences, Merck, Sinovac Biotech, BPL, Bio-Med, and Changchun Changsheng Life Sciences Limited. Key players in the varicella vaccine market are leveraging a range of strategic initiatives to strengthen their market presence. A major focus is on expanding production capacity and improving cold-chain logistics to ensure consistent global supply. Companies are also investing in research and development to create combination vaccines that integrate varicella protection into broader immunization schedules, enhancing convenience and compliance. Strategic collaborations with governments and global health agencies are facilitating broader distribution, especially in low- and middle-income countries.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Vaccine type
- 2.2.3 Application
- 2.2.4 Age group
- 2.2.5 Procurement
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of chickenpox
- 3.2.1.2 Growing immunization programs by the government
- 3.2.1.3 Technological advancements in vaccine development
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory requirements
- 3.2.2.2 High cost of storage and transportation of vaccine
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of National Immunization Programs
- 3.2.3.2 Rising demand for adult and adolescent vaccination
- 3.2.3.3 Growth in combination vaccines
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Future market trends
- 3.7 Pipeline analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vaccine Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Monovalent varicella vaccine
- 5.3 Combination varicella vaccine
Chapter 6 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Chickenpox immunization
- 6.3 Mumps, measles, rubella, and varicella (MMRV) immunization
Chapter 7 Market Estimates and Forecast, By Age Group, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pediatrics
- 7.3 Adolescents and adults
Chapter 8 Market Estimates and Forecast, By Procurement, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Public
- 8.3 Private
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Italy
- 9.3.5 Spain
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Bio-Med
- 10.2 Changchun Changsheng Life Sciences Limited
- 10.3 GC Pharma (Green Cross Holdings)
- 10.4 GlaxoSmithKline
- 10.5 Merck
- 10.6 Mitsubishi Tanabe Pharma Corporation
- 10.7 Novo Medi Sciences
- 10.8 Sanofi
- 10.9 Seqirus
- 10.10 Sinovac Biotech
- 10.11 SK Bioscience
- 10.12 Takeda Pharmaceutical Company Limited













