|
시장보고서
상품코드
1766327
수두증 션트 시장(2025-2035년) : 기회, 성장 촉진요인, 산업 동향 분석, 예측Hydrocephalus Shunt Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2035 |
||||||
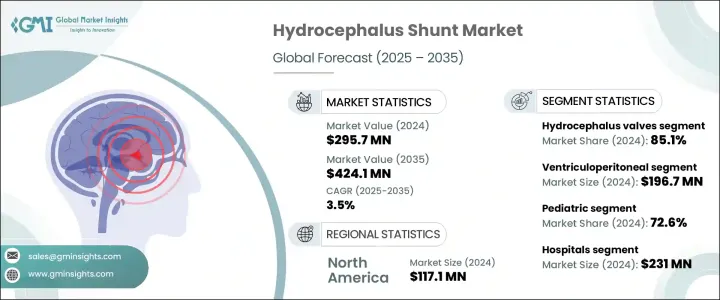
세계의 수두증 션트 시장은 2024년에는 2억 9,570만 달러에 달하였고, CAGR 3.5%로 성장하여 2035년에는 4억 2,410만 달러에 이를 것으로 예측됩니다.
이 성장의 주요 요인은 외상성 뇌 손상과 뇌종양과 관련된 수두증과 관련된 증례 수 증가입니다. 치료 기술의 진보도 시장에 큰 영향을 미치고 있습니다. 또한 압력 조절 가능한 션트 밸브, 사이펀 방지 시스템, 생체 적합 재료의 사용 등의 개발로 합병증을 최소화하면서 환자의 예후를 향상시키고 있습니다.
실시간 뇌척수액 모니터링과 유량 조절을 가능하게 하는 스마트 션트 시스템의 도입은 외과적 재수술의 필요성을 더욱 줄이고 있습니다. 수두증 션트는 과도한 뇌척수액을 뇌실에서 체내의 다른 부위로 이동 및 흡수시켜 두개내압의 상승과 잠재적인 신경장애를 방지하도록 설계되어 있습니다.
수두증 밸브 부문은 2024년에 85.1%를 차지했으며, 이러한 성장은 수두증 환자 증가와 세계적인 관련 수술의 급증으로 인한 것입니다. 비침습적 압력 조절 기술을 통해 환자 관리가 개선되었고, 많은 지역에서 발생하는 고령자 증가나 출생율의 상승이 수두증 환자 증가에 기여하고 있습니다.
심실 복막 션트는 2024년에 1억 9,670만 달러를 차지했습니다. 심실 복막 션트는 비용 효과, 수술 적응성 및 다양한 임상 환경에서 일관된 성공으로 인해 수두증 치료에 바람직한 접근법입니다. 소아 의료나 헬스케어 예산이 한정되어 있는 지역에서 보다 저렴한 가격으로 널리 채용되고 있습니다.
미국 수두증 션트 시장은 2034년까지 1억 4,630만 달러에 이를 것으로 예상되고 있습니다. 지역 시장은 프로그램 가능한 설계, 보다 우수한 뇌척수액 조정법 등의 기술 혁신에 의해 지원되고 있습니다. 이러한 개발은 치료 성적을 향상시킬 뿐만 아니라, 재수술의 빈도를 감소시킵니다. 또한, 연구 개발에 대한 지속적인 투자와 선진적인 제품의 출시로 이 지역 전체 시장의 성장 궤도를 상승시킬 것으로 예측됩니다.
수두증 션트 영역의 주요 시장 진출기업으로는 Medtronic, B. Braun, Integra LifeSciences Holdings, Sophysa, Luciole Medical, DESU, HLL Lifecare, Hpbio, Kaneka Medix, G. Surgiwear, Wellong Instruments Co., and Neutral Pharma Companies 등이 있습니다. 수두증 션트 시장에서 사업을 전개하는 기업은 시장에서의 지위를 강화하기 위해 기술 혁신, 연구 개발 투자, 세계 전개에 주력하고 있습니다.
주요 전략은 첨단 프로그래머블 밸브, 항균 션트 코팅, 실시간 모니터링 기능을 갖춘 스마트 션트 시스템 개발 등이 있습니다. 또한 기업은 병원과 뇌신경 외과 센터와 전략적 제휴를 맺어 임상 피드백 루프를 개선하고 차세대 장비의 공동 개발을 추진하고 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- 선천성 수두증의 유병률 증가
- 수두증의 외과적 치료에 대한 의식 증가
- 수두증 치료의 보험 환급 적용 유무
- 고령화 인구 증가
- 업계의 잠재적 위험 및 과제
- 신흥국에서 복잡한 뇌신경외과 수술을 실시하기 위한 인프라의 부족
- 수두증 션트 수술과 그 후의 케어에 관련된 고액의 비용
- 시장 기회
- 기술의 진보와 스마트 션트의 혁신
- 인공지능(AI)과 첨단 진단의 통합
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 미국
- 유럽
- 기술과 혁신의 상황
- Porter's Five Forces 분석
- PESTEL 분석
- 장래 시장 동향
- 특허 분석
제4장 경쟁 구도
- 소개
- 경쟁 시장 점유율 분석
- 기업 매트릭스 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
제5장 시장 추계 및 예측 : 제품 유형별(2021-2035년)
- 주요 동향
- 수두증 밸브
- 조정 압력 밸브
- 고정 압력 밸브
- 수두증 카테터
- 표준 카테터
- 항생제 카테터
제6장 시장추계 및 예측 : 처치 유형별(2021-2035년)
- 주요 동향
- 뇌실 복강
- 허리 복막
- 심실 방실
- 뇌실 흉막
제7장 시장추계 및 예측 : 연령별(2021-2035년)
- 주요 동향
- 소아
- 성인
제8장 시장 추계 및 예측 : 최종 용도별(2021-2035년)
- 주요 동향
- 병원
- 외래수술센터(ASC)
제9장 시장추계 및 예측 : 지역별(2021-2035년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 네덜란드
- 아시아태평양
- 일본
- 중국
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- B. Braun
- DESU
- G. Surgiwear
- HLL Lifecare
- Hpbio
- Integra LifeSciences Holdings
- Kaneka Medix
- Luciole Medical
- Medtronic
- Neutral Pharma
- Sophysa
- Wellong Instruments Co.
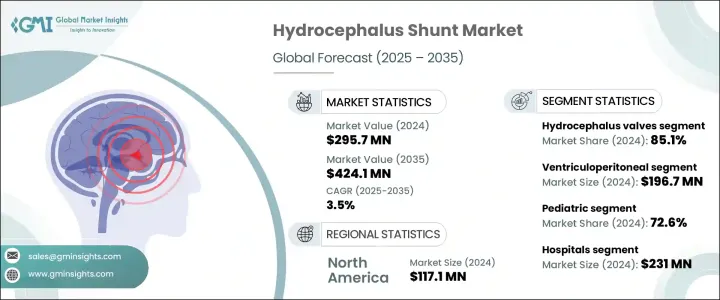
The Global Hydrocephalus Shunt Market was valued at USD 295.7 million in 2024 and is estimated to grow at a CAGR of 3.5% to reach USD 424.1 million by 2035. This growth is primarily driven by the rising number of cases related to hydrocephalus, frequently linked to traumatic brain injuries and brain tumors. As the prevalence of these conditions increases, so does the demand for reliable treatment solutions. Advances in medical technology have also significantly impacted this market. Developments such as adjustable pressure shunt valves, anti-siphon systems, and the use of biocompatible materials are enhancing patient outcomes while minimizing complications.
The introduction of smart shunt systems, which allow real-time cerebrospinal fluid monitoring and flow regulation, is further reducing the need for surgical revisions. Major manufacturers like Integra LifeSciences Holdings, Medtronic, and B. Braun continue to invest in research and product innovation to meet evolving clinical needs. Hydrocephalus shunts are designed to divert excess cerebrospinal fluid from the brain's ventricles to other areas of the body for absorption, preventing elevated intracranial pressure and potential neurological damage. The medical community increasingly relies on these systems for both pediatric and adult hydrocephalus management, further reinforcing their importance in modern neurosurgical practice.
The hydrocephalus valves segment led the market in 2024, capturing 85.1%. This growth stems from the increasing frequency of hydrocephalus cases and a surge in related surgeries worldwide. Innovative valve designs offering programmable control and antimicrobial properties are playing a crucial role in reducing complications and revision rates. Non-invasive pressure adjustments have improved patient management, while the rising number of elderly individuals and higher birth rates in many regions contribute to the growing hydrocephalus patient population. Surgeons and healthcare providers are increasingly choosing valves that offer long-term durability and personalized control over CSF drainage, especially in specialized medical facilities.
Ventriculoperitoneal shunts accounted for USD 196.7 million in 2024. VP shunting continues to be the preferred approach for treating hydrocephalus due to its cost-effectiveness, surgical adaptability, and consistent success across various clinical settings. Compared to alternative treatments, VP shunts offer a more affordable and widely adopted solution, particularly in pediatric care and regions with limited healthcare budgets. The rising number of cases involving congenital hydrocephalus in children and the growing recognition of normal pressure hydrocephalus among older adults are fueling the increased deployment of VP systems across hospitals globally.
United States Hydrocephalus Shunt Market is expected to reach USD 146.3 million by 2034. With over a million individuals affected nationwide and tens of thousands of shunt placements performed annually, the condition remains a significant medical challenge. Market expansion is supported by innovations in programmable shunts, improved device designs, and better cerebrospinal fluid regulation methods. These developments not only improve treatment outcomes but also decrease the frequency of revision procedures. Additionally, sustained investment in R&D and advanced product launches are expected to elevate the market's trajectory across the region.
Leading market participants in the Hydrocephalus Shunt Space include Medtronic, B. Braun, Integra LifeSciences Holdings, Sophysa, Luciole Medical, DESU, HLL Lifecare, Hpbio, Kaneka Medix, G. Surgiwear, Wellong Instruments Co., and Neutral Pharma. Companies operating in the hydrocephalus shunt market are focusing on innovation, R&D investment, and global expansion to reinforce their market positions.
Key strategies include developing advanced programmable valves, antimicrobial shunt coatings, and smart shunt systems with real-time monitoring features. These innovations aim to minimize revision surgeries and enhance patient safety. Firms are also entering strategic collaborations with hospitals and neurosurgery centers to improve clinical feedback loops and co-develop next-gen devices. Regulatory approvals and strong distribution networks across emerging economies are other priorities, enabling companies to tap into high-growth regions.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product type trends
- 2.2.3 Procedure type trends
- 2.2.4 Age group trends
- 2.2.5 End use trends
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of congenital hydrocephalus
- 3.2.1.2 Growing awareness towards surgical treatment of hydrocephalus
- 3.2.1.3 Availability of reimbursement coverage for hydrocephalus treatments
- 3.2.1.4 Increasing geriatric population
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Lack of infrastructure for performing complex neurosurgeries in emerging countries
- 3.2.2.2 High cost associated to hydrocephalus shunt surgery and follow-up care
- 3.2.3 Market opportunities
- 3.2.3.1 Technological Advancements and Smart Shunt Innovation
- 3.2.3.2 Integration of Artificial Intelligence (AI) and Advanced Diagnostics
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.3.1 By product type
- 3.3.2 By procedure type
- 3.3.3 By age group
- 3.3.4 By end use
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.5 Technology and innovation landscape
- 3.6 Porter's analysis
- 3.7 PESTEL analysis
- 3.8 Future market trends
- 3.9 Patent analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.1.1 Medtronic
- 4.1.2 Integra LifeSciences Holdings
- 4.1.3 B. Braun
- 4.2 Competitive market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Hydrocephalus valves
- 5.2.1 Adjusted pressure valves
- 5.2.2 Fixed Pressure valves
- 5.3 Hydrocephalus catheter
- 5.3.1 Standard catheters
- 5.3.2 Antibiotic catheters
Chapter 6 Market Estimates and Forecast, By Procedure Type, 2021 – 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Ventriculoperitoneal
- 6.3 Lumboperitoneal
- 6.4 Ventriculoatrial
- 6.5 Ventriculopleural
Chapter 7 Market Estimates and Forecast, By Age Group, 2021 – 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Pediatric
- 7.3 Adult
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2035 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Ambulatory surgical centers
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2035 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Italy
- 9.3.5 Spain
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 Japan
- 9.4.2 China
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 B. Braun
- 10.2 DESU
- 10.3 G. Surgiwear
- 10.4 HLL Lifecare
- 10.5 Hpbio
- 10.6 Integra LifeSciences Holdings
- 10.7 Kaneka Medix
- 10.8 Luciole Medical
- 10.9 Medtronic
- 10.10 Neutral Pharma
- 10.11 Sophysa
- 10.12 Wellong Instruments Co.