
|
시장보고서
상품코드
1775084
장기 보존 시장 : 보존액 유형별, 기술별, 장기별, 최종사용자별 - 예측(-2030년)Organ Preservation Market by Solution Type (UW, Custodial HTK, Perfadex), Technique (Static Cold Storage, Hypothermic, Normothermic), Organ (Kidneys, Liver, Heart), End User (Transplant Centers, Hospitals, Specialty Clinics) - Global Forecast to 2030 |
||||||
세계의 장기 보존 시장 규모는 2025년 20만 달러에서 예측 기간 동안 CAGR 6.8%로 성장하여 2030년까지 3억 달러에 달할 것으로 예측됩니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2030년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2024-2030년 |
| 단위 | 10억 달러 |
| 부문 | 보존액, 기술, 장기 유형, 최종사용자 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카, GCC 국가 |
장기 보존액 및 장치의 주요 목적은 장기 조달에서 이식까지 장기의 생존 능력과 기능을 유지하는 것입니다. 전 세계 장기 이식 건수 증가, 보존 기술 발전, 저체온 및 정상체온 기계 관류 시스템과 같은 특수 보존 솔루션의 도입 등 여러 요인이 이 시장의 지속적인 성장을 견인하고 있습니다. 말기 신부전, 간부전 등 만성질환의 발병률 증가는 장기 이식에 대한 수요를 촉진하고 있으며, 이에 따라 효과적인 보존 기술에 대한 요구가 증가하고 있습니다.
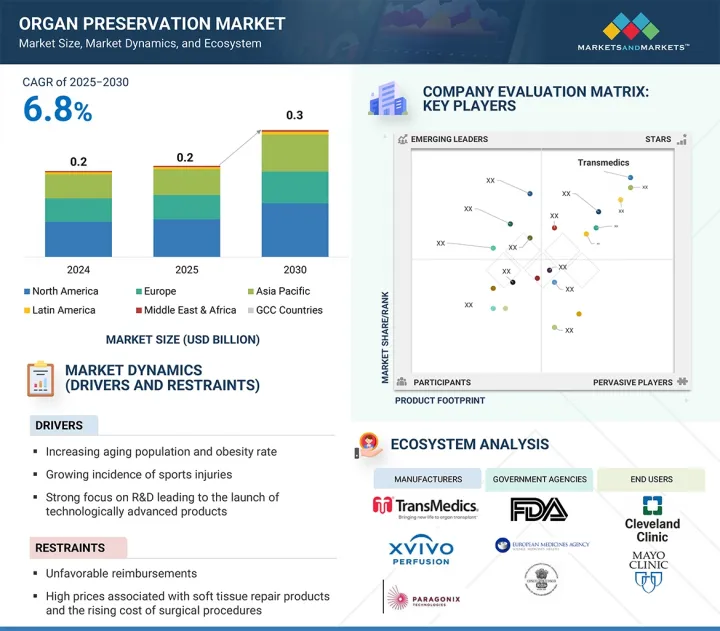
보존액 유형별로는 2024년 위스콘신대학교(UW) 부문이 가장 큰 시장 점유율을 차지했습니다.
시장은 보존액 유형별로 UW, Custodiol HTK, Perfadex, 기타 보존액으로 분류되며, 2024년 위스콘신대학교(UW) 보존액이 간, 신장, 췌장과 같은 장기 보존에 효과적임이 입증되는 등 높은 채택률과 특성으로 가장 큰 시장 점유율을 차지했습니다. UW 보존액은 최초로 도입된 세포 내 장기 보존액으로 장기 보존의 세계 표준으로 널리 인정받고 있습니다. 그 확립된 임상적 성과와 신뢰성으로 인해 이식 외과의사 및 의료 시설에서 선호되는 선택으로 자리매김하고 있습니다.
기술별로는 정상체온 기계 관류 부문이 예측 기간 동안 가장 높은 CAGR을 기록할 것으로 예상됩니다.
정상체온 기계 관류 부문은 예측 기간 동안 가장 높은 CAGR로 성장할 것으로 예상됩니다. 이 기술은 정상적인 체온을 유지하고 장기에 필수 영양소와 산소를 공급함으로써 자연 생리적 조건을 시뮬레이션합니다. 보존 기간 동안 세포 기능과 신진대사를 지원합니다. 정상체온 관류는 장기의 생존 능력을 유지하고, 특히 간 이식에서 염증 반응과 허혈성 손상을 최소화하는 데 도움이 됩니다. 정상체온 관류는 이식편의 재생을 향상시키는 데 필수적이며, 고령자나 합병증을 가진 사람 등 확대된 기준 기증자와 관련된 이식 사례에서 좋은 결과와 관련이 있습니다. 그 결과, 이식편의 장기 생존과 기능을 최적화하고자 하는 이식 전문가들 사이에서 그 중요성이 커지고 있습니다.
장기 유형별로는 폐 부문이 예측 기간 동안 가장 높은 CAGR로 성장할 것으로 예상됩니다.
폐 부문은 예측 기간 동안 가장 높은 CAGR로 성장할 것으로 예상됩니다. 폐 이식은 만성폐쇄성폐질환(COPD), 특발성 폐섬유증, 낭포성섬유증과 같은 말기 폐질환 환자에게 선호되는 치료법이며, 이는 모두 이 부문의 성장에 기여하고 있습니다. 성인 환자에서 말기 폐질환의 다른 흔한 원인으로는 알파1 항트립신 결핍증, 폐고혈압 등이 있습니다. 호흡기질환이 증가함에 따라 폐 이식이 필요한 환자 수가 증가할 것으로 예상되며, 이는 폐 이식에 대한 수요를 촉진하고 있습니다. 또한, 이식편 생존율과 장기 이식편 기능의 발전으로 폐 이식은 중요하고 종종 생명을 구할 수 있는 치료 옵션으로 자리매김하고 있습니다.
최종사용자별로는 전문 클리닉 부문이 예측 기간 동안 가장 높은 CAGR로 성장할 것으로 예상됩니다.
장기 보존 시장은 최종사용자에 따라 장기 이식 센터, 병원, 전문 클리닉으로 구분됩니다. 장기 보존 시장에서 전문 클리닉 부문은 이식 수술 및 수술 후 관리에서 전문 클리닉의 역할이 증가함에 따라 가장 높은 CAGR로 성장할 것으로 예상됩니다. 전문 클리닉은 장기 보존에 필요한 집중적인 전문 지식, 짧은 시간, 첨단 기술을 제공합니다. 또한, 전문적인 치료에 대한 환자 선호도 증가와 민간 의료 투자 증가는 이 부문의 성장을 더욱 가속화할 것으로 예상됩니다.
지역별로는 아시아태평양이 예측 기간 동안 가장 높은 CAGR로 성장할 것으로 예상됩니다.
아시아태평양은 예측 기간 동안 가장 높은 CAGR로 성장할 것으로 예상됩니다. 이 부문의 높은 성장률은 주로 유리한 정부 정책, 사회적 인식 개선, 장기 기증 건수 증가의 조합에 기인합니다. 예를 들어, 중국에서는 시민 교육 강화, 동원 활동, 장기 이식 기술의 발전으로 장기 기증률이 향상되고 있습니다. 한편, 인도는 의료 인프라의 급속한 발전이 시장 성장을 뒷받침하고 의료관광을 촉진하고 있습니다. 일본에서는 종교적 우려에도 불구하고 장기 기증에 대한 의욕이 높아져 장기 기증률이 개선되고 있습니다.
세계의 장기 보존 시장에 대해 조사 분석했으며, 주요 촉진요인과 억제요인, 경쟁 상황, 향후 동향 등의 정보를 전해드립니다.
목차
제1장 소개
제2장 조사 방법
제3장 주요 요약
제4장 중요한 인사이트
- 장기 보존 시장의 기업에서 매력적인 기회
- 아시아태평양의 장기 보존 시장 : 보존액별, 국가별
- 장기 보존 시장 : 지리적 성장 기회
- 장기 보존 시장 : 지역별(2023-2030년)
- 장기 보존 시장 : 선진 시장과 신흥 시장
제5장 시장 개요
- 소개
- 시장 역학
- 성장 촉진요인
- 성장 억제요인
- 기회
- 과제
- 생태계 분석
- 밸류체인 분석
- 가격 분석
- 공급망 분석
- 특허 분석
- 장기 보존 시장의 특허 공보 동향
- 장기 보존 특허의 주요 출원 기업
- 관할 분석 : 장기 보존 시장 특허의 주요 출원국
- 기술 분석
- 주요 기술
- 인접 기술
- 보완 기술
- 무역 분석
- 수입 데이터
- 수출 데이터
- 주요 회의와 이벤트(2025-2026년)
- 규제 상황
- 규제 분석
- 규제기관, 정부기관, 기타 조직
- Porter's Five Forces 분석
- 주요 이해관계자와 구입 기준
- 고객 비즈니스에 영향을 미치는 동향/혼란
- 사례 연구 분석
- 투자와 자금 조달 시나리오
- 상환 시나리오
- 장기 보존 시장에 대한 AI의 영향
- 장기 보존 시장의 최종사용자 기대
- 2025년 미국 관세의 영향
- 소개
- 주요 관세율
- 가격의 영향 분석
- 최종사용자에 대한 영향
제6장 장기 보존 시장 : 보존액별
- 소개
- UW
- Custodiol HTK
- Perfadex
- 기타 보존액
제7장 장기 보존 시장 : 기술별
- 소개
- 정적 냉장 보존
- 저체온 기계 관류
- 정상체온 기계 관류
제8장 장기 보존 시장 : 장기별
- 소개
- 신장
- 간
- 폐
- 심장
- 기타 장기
제9장 장기 보존 시장 : 최종사용자별
- 소개
- 장기 이식 센터
- 병원
- 전문 클리닉
제10장 장기 보존 시장 : 지역별
- 소개
- 북미
- 거시경제 전망
- 미국
- 캐나다
- 유럽
- 거시경제 전망
- 독일
- 프랑스
- 영국
- 스페인
- 이탈리아
- 기타 유럽
- 아시아태평양
- 거시경제 전망
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 라틴아메리카
- 거시경제 전망
- 브라질
- 멕시코
- 기타 라틴아메리카
- 중동 및 아프리카
- GCC 국가
제11장 경쟁 구도
- 개요
- 주요 진출 기업의 전략/강점
- 매출 분석(2022-2024년)
- 시장 점유율 분석
- 기업 평가 매트릭스 : 주요 기업(2024년)
- 기업 평가 매트릭스 : 스타트업/중소기업(2024년)
- 기업 평가와 재무 지표
- 브랜드/제품의 비교
- 경쟁 시나리오
제12장 기업 개요
- 주요 기업
- PARAGONIX TECHNOLOGIES, INC.
- XVIVO PERFUSION AB
- DR. FRANZ KOHLER CHEMIE GMBH
- ESSENTIAL PHARMACEUTICALS, LLC(SUBSIDIARY OF ACCORD HEALTHCARE)
- TRANSMEDICS, INC.
- ORGANOX LIMITED
- 21ST CENTURY MEDICINE
- BIOLIFE SOLUTIONS, INC.
- BRIDGE TO LIFE LIMITED
- WATERS MEDICAL SYSTEMS LLC
- 기타 기업
- SHANGHAI GENEXT MEDICAL TECHNOLOGY CO., LTD.
- PRESERVATION SOLUTIONS, INC.
- CARNAMEDICA
- TRANSPLANT BIOMEDICALS
- INSTITUT GEORGES LOPEZ
- GLOBAL TRANSPLANT SOLUTIONS
- AVIONORD
- ORGAN PRESERVATION SOLUTIONS LTD.
- EBERS
- S.A.L.F.
- BIOCHEFA
- VASCULAR PERFUSION SOLUTIONS, INC.
- TX INNOVATIONS
- TAIYO NIPPON SANSO CORPORATION
- X-THERMA, INC.
제13장 부록
ksm 25.07.29The global organ preservation market is projected to reach USD 0.3 billion by 2030 from USD 0.2 million in 2025, at a CAGR of 6.8% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2024-2030 |
| Units Considered | Value (USD billion) |
| Segments | Solution, Technique, Organ Type, and End-user |
| Regions covered | North America, Europe, Asia Pacific, Latin America, the Middle East & Africa, and GCC Countries |
The primary objective of organ preservation solutions and devices is to maintain the viability and functionality of organs between procurement and transplantation. Several factors fuel consistent growth in this market, including the increasing volume of organ transplants globally, technological advancements in preservation techniques, and the introduction of specialized solutions such as hypothermic and normothermic machine perfusion systems. The growing incidence of chronic diseases such as end-stage renal and liver failure is driving the demand for organ transplants, which in turn boosts the need for effective preservation technologies.
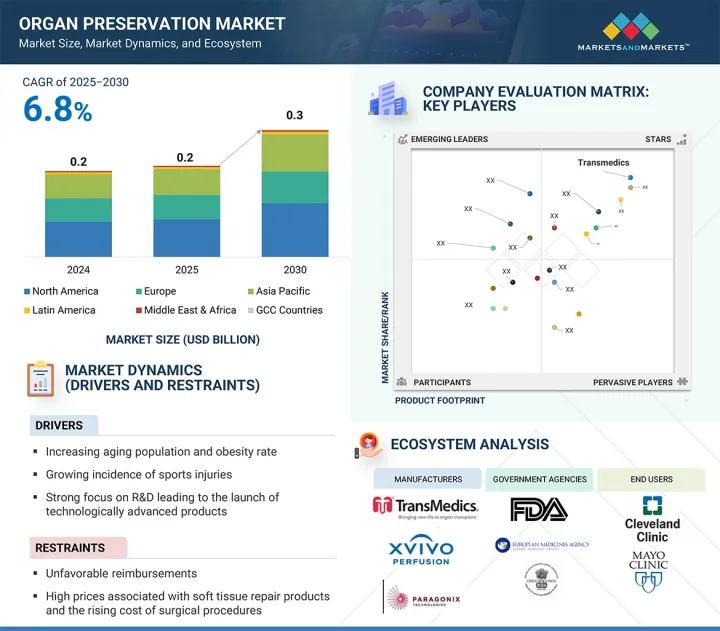
By solution type, the University of Wisconsin (UW) segment accounted for the largest market share in 2024.
The market is categorized into UW, Custodiol HTK, Perfadex, and other solutions by solution type. In 2024, the University of Wisconsin (UW) solution accounted for the largest market share owing to high adoption and features such as proven efficacy in preserving organs like the liver, kidneys, and pancreas. It was the first introduced intracellular preservation solution and is widely regarded as the global benchmark for organ preservation. Its established clinical outcomes and reliability have solidified its position as the preferred choice among transplant surgeons and healthcare facilities.
By technique, the normothermic machine perfusion segment is expected to register the highest CAGR during the forecast period.
The normothermic machine perfusion segment is expected to grow at the highest CAGR during the forecast period. This technique simulates natural physiological conditions by maintaining normal body temperature and supplying essential nutrients and oxygen to the organ. It supports cellular function and metabolism throughout the preservation period. Normothermic perfusion helps maintain organ viability and minimizes inflammatory responses and ischemic injury, particularly in liver transplants. It is vital in improving graft regeneration and has been associated with better outcomes in transplant cases involving extended criteria donors, such as older individuals or those with comorbidities. As a result, its relevance is growing among transplant professionals seeking to optimize long-term graft survival and function.
By organ type, the lungs segment is expected to grow at the highest CAGR during the forecast period.
The lungs segment is expected to grow at the highest CAGR during the forecast period. Lung transplantation is regarded as the preferred treatment for patients with end-stage lung diseases such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, or cystic fibrosis, all of which contribute to the growth of this segment. Other common causes of end-stage lung disease in adult patients include alpha-1 antitrypsin deficiency and pulmonary hypertension. With the increasing prevalence of respiratory illnesses, the number of patients requiring lung transplants is anticipated to rise, thereby driving demand for lung transplants. Additionally, advancements in graft survival and long-term graft function have established lung transplantation as a vital and often life-saving therapeutic option.
By end user, the specialty clinics segment is expected to grow at the highest CAGR during the forecast period.
The organ preservation market is segmented by end-users into organ transplant centers, hospitals, and specialty clinics. The specialty clinics segment is projected to grow at the highest CAGR in the organ preservation market due to their increasing role in transplant procedures and post-operative care. These clinics offer focused expertise, faster turnaround times, and advanced technologies tailored to organ preservation needs. Additionally, growing patient preference for specialized care and the rise in private healthcare investments further accelerate their growth in this segment.
By region, the Asia Pacific is expected to grow at the highest CAGR during the forecast period.
The Asia Pacific region is projected to grow at the highest CAGR during the forecast period. The high growth rate of this segment is primarily attributed to the combination of favorable government policies, increased public awareness, and a higher number of organ donations. For instance, China has seen its donation rates improve thanks to intensified public education, mobilization efforts, and technological advancements in organ transplants. Meanwhile, India's rapid development in healthcare infrastructure has supported market growth and fostered medical tourism. Even with religious considerations, Japan has observed an improvement in organ donation due to a higher willingness to donate organs.
The breakdown of primary participants was as mentioned below:
- By Company Type - Tier 1-35%, Tier 2-45%, and Tier 3-20%
- By Designation - C-level-35%, Director-level-25%, Others-40%
- By Region - North America-45%, Europe-30%, Asia Pacific-20%, Latin America- 3%, the Middle East & Africa-1%, and GCC countries-1%
Key Players in the Organ Preservation Market
The key players operating in the organ preservation market include Paragonix Technologies (US), XVIVO Perfusion AB (Sweden), Dr. Franz Kohler Chemie GmbH (Germany), Essential Pharmaceuticals, LLC (US), TransMedics (US), OrganOx Limited (UK), 21st Century Medicine (US), Shanghai Genext Medical Technology (China), Bridge to Life Limited (US), Waters Medical Systems (US), Preservation Solutions (US), Carnamedica (Poland), Transplant Biomedicals (Spain), Institut Georges Lopez (France), Global Transplant Solutions (US), Avionord (Italy), Organ Preservation Solutions (England), EBERS (Spain), S.A.L.F. (Italy), Biochefa (Poland), Vascular Perfusion Solutions (US), and TX Innovations (Netherlands).
Research Coverage:
The report analyzes the organ preservation market and aims to estimate the market size and future growth potential based on various segments such as solution, technique, organ type, end user, and region. The report also includes a product portfolio matrix of various organ preservation products available in the market. The report also provides a competitive analysis of the key players in this market, along with their company profiles, product offerings, and key market strategies.
Reasons to Buy the Report
The report will enrich established firms and new entrants/smaller firms to gauge the market's pulse, which in turn would help them garner a more significant share of the market. Firms purchasing the report could use one or any combination of the below-mentioned strategies to strengthen their position in the market.
This report provides insights into the following pointers:
Analysis of key drivers (Ascending cases of multiple organ failure and growth in the geriatric population, the increasing initiatives to promote public awareness and encourage organ donation, the rising number of organ donors boost adoption of solid organ transplantation procedures); restraints (High cost of organ transplantation and religious concerns and misconceptions associated to organ donation); opportunities (the increasing focus on healthcare investments); and challenges (Significant gap between the number of organs donated and organs required annually coupled with the development of artificial organs)
- Market Penetration: Comprehensive information on product portfolios offered by the top players in the global organ preservation market. The report analyzes this market by solution, technique, organ type, and end user.
- Product Enhancement/Innovation: Detailed insights on upcoming trends and product launches in the global organ preservation market
- Market Development: Comprehensive information on the lucrative emerging markets by solution type, technique, organ type, and end user
- Market Diversification: Exhaustive information about new products or product enhancements, growing geographies, recent developments, and investments in the global organ preservation market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings, competitive leadership mapping, and capabilities of leading players in the global organ preservation market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.2.1 INCLUSIONS AND EXCLUSIONS
- 1.3 MARKET SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH APPROACH
- 2.2 RESEARCH DESIGN
- 2.2.1 SECONDARY RESEARCH
- 2.2.1.1 Key data from secondary sources
- 2.2.2 PRIMARY DATA
- 2.2.2.1 Key data from primary sources
- 2.2.2.2 Key industry insights
- 2.2.1 SECONDARY RESEARCH
- 2.3 MARKET SIZE ESTIMATION
- 2.4 MARKET BREAKDOWN & DATA TRIANGULATION
- 2.5 MARKET RANKING ANALYSIS
- 2.6 RESEARCH ASSUMPTIONS
- 2.7 RESEARCH LIMITATIONS
- 2.7.1 METHODOLOGY-RELATED LIMITATIONS
- 2.7.2 SCOPE-RELATED LIMITATIONS
- 2.8 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 ATTRACTIVE OPPORTUNITIES FOR PLAYERS IN ORGAN PRESERVATION MARKET
- 4.2 ASIA PACIFIC: ORGAN PRESERVATION MARKET, BY SOLUTION AND COUNTRY
- 4.3 ORGAN PRESERVATION MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.4 ORGAN PRESERVATION MARKET, BY REGION, 2023-2030
- 4.5 ORGAN PRESERVATION MARKET: DEVELOPED VS. EMERGING MARKETS
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Growing geriatric population and subsequent growth in cases of multiple organ failure
- 5.2.1.2 Increasing initiatives to promote public awareness and encourage organ donation
- 5.2.1.3 Rising number of organ donors and growing adoption of solid organ transplantation procedures
- 5.2.2 RESTRAINTS
- 5.2.2.1 High cost of organ transplantation
- 5.2.2.2 Religious concerns and misconceptions associated with organ donation
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growing healthcare investments
- 5.2.4 CHALLENGES
- 5.2.4.1 Significant gap between number of organs donated and organs required annually
- 5.2.4.2 Development of artificial organs
- 5.2.1 DRIVERS
- 5.3 ECOSYSTEM ANALYSIS
- 5.4 VALUE CHAIN ANALYSIS
- 5.5 PRICING ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 PATENT ANALYSIS
- 5.7.1 PATENT PUBLICATION TRENDS FOR ORGAN PRESERVATION MARKET
- 5.7.2 TOP APPLICANTS (COMPANIES) OF ORGAN PRESERVATION PATENTS
- 5.7.3 JURISDICTION ANALYSIS: TOP APPLICANTS (COUNTRIES) FOR PATENTS IN ORGAN PRESERVATION MARKET
- 5.8 TECHNOLOGY ANALYSIS
- 5.8.1 KEY TECHNOLOGIES
- 5.8.1.1 Machine perfusion
- 5.8.1.2 Organ perfusion
- 5.8.2 ADJACENT TECHNOLOGIES
- 5.8.2.1 Cryopreservation & vitrification
- 5.8.3 COMPLEMENTARY TECHNOLOGIES
- 5.8.3.1 Temperature monitoring devices
- 5.8.1 KEY TECHNOLOGIES
- 5.9 TRADE ANALYSIS
- 5.9.1 IMPORT DATA
- 5.9.2 EXPORT DATA
- 5.10 KEY CONFERENCES AND EVENTS IN 2025-2026
- 5.11 REGULATORY LANDSCAPE
- 5.11.1 REGULATORY ANALYSIS
- 5.11.1.1 North America
- 5.11.1.1.1 US
- 5.11.1.1.2 Canada
- 5.11.1.2 Europe
- 5.11.1.3 Asia Pacific
- 5.11.1.3.1 China
- 5.11.1.3.2 Japan
- 5.11.1.3.3 India
- 5.11.1.4 Latin America
- 5.11.1.4.1 Brazil
- 5.11.1.4.2 Mexico
- 5.11.1.5 Middle East
- 5.11.1.6 Africa
- 5.11.1.1 North America
- 5.11.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.11.1 REGULATORY ANALYSIS
- 5.12 PORTER'S FIVE FORCES ANALYSIS
- 5.12.1 THREAT FROM NEW ENTRANTS
- 5.12.2 THREAT FROM SUBSTITUTES
- 5.12.3 BARGAINING POWER OF SUPPLIERS
- 5.12.4 BARGAINING POWER OF BUYERS
- 5.12.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.13 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.13.2 BUYING CRITERIA
- 5.14 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.15 CASE STUDY ANALYSIS
- 5.16 INVESTMENT AND FUNDING SCENARIO
- 5.17 REIMBURSEMENT SCENARIO
- 5.18 IMPACT OF AI ON ORGAN PRESERVATION MARKET
- 5.19 END USER EXPECTATIONS IN ORGAN PRESERVATION MARKET
- 5.20 IMPACT OF 2025 US TARIFFS
- 5.20.1 INTRODUCTION
- 5.20.2 KEY TARIFF RATES
- 5.20.3 PRICE IMPACT ANALYSIS
- 5.20.4 IMPACT ON END USERS
6 ORGAN PRESERVATION MARKET, BY SOLUTION
- 6.1 INTRODUCTION
- 6.2 UNIVERSITY OF WISCONSIN
- 6.2.1 METABOLICALLY INERT SUBSTRATES ENABLE BETTER TRANSPLANTATION OUTCOMES
- 6.3 CUSTODIOL HTK
- 6.3.1 INDICATED FOR PERFUSION AND FLUSHING OF KIDNEYS, LIVER, PANCREAS, AND HEART
- 6.4 PERFADEX
- 6.4.1 LAUNCH OF PERFADEX PLUS TO DRIVE MARKET
- 6.5 OTHER SOLUTIONS
7 ORGAN PRESERVATION MARKET, BY TECHNIQUE
- 7.1 INTRODUCTION
- 7.2 STATIC COLD STORAGE
- 7.2.1 MOST WIDELY ADOPTED ORGAN PRESERVATION TECHNIQUE
- 7.3 HYPOTHERMIC MACHINE PERFUSION
- 7.3.1 ADVANTAGES ASSOCIATED WITH HYPOTHERMIC MACHINE PERFUSION TO DRIVE DEMAND
- 7.4 NORMOTHERMIC MACHINE PERFUSION
- 7.4.1 PROVIDES VIABLE ENVIRONMENT FOR ORGAN PRESERVATION, VIABILITY, AND REPAIR PRIOR TO TRANSPLANTATION
8 ORGAN PRESERVATION MARKET, BY ORGAN TYPE
- 8.1 INTRODUCTION
- 8.2 KIDNEYS
- 8.2.1 MOST TRANSPLANTED ORGANS GLOBALLY
- 8.3 LIVER
- 8.3.1 RISING PREVALENCE OF LIVER DISEASE AND GROWING CASES OF LIVER FAILURE TO DRIVE DEMAND FOR LIVER TRANSPLANTATION PROCEDURES
- 8.4 LUNGS
- 8.4.1 GROWTH IN COPD INCIDENCE TO BOOST DEMAND FOR PRESERVATION TECHNIQUES AND PRODUCTS
- 8.5 HEART
- 8.5.1 ADVANCEMENTS IN HEART PRESERVATION TECHNIQUES TO DRIVE MARKET
- 8.6 OTHER ORGANS
9 ORGAN PRESERVATION MARKET, BY END USER
- 9.1 INTRODUCTION
- 9.2 ORGAN TRANSPLANT CENTERS
- 9.2.1 MAJOR END USERS OF ORGAN PRESERVATION SOLUTIONS
- 9.3 HOSPITALS
- 9.3.1 INCREASING CASES OF ACCIDENTAL INJURIES AND CHRONIC DISEASES TO DRIVE MARKET
- 9.4 SPECIALTY CLINICS
- 9.4.1 INCREASING PREVALENCE OF END-STAGE DISEASES AND ORGAN FAILURE TO DRIVE MARKET
10 ORGAN PRESERVATION MARKET, BY REGION
- 10.1 INTRODUCTION
- 10.2 NORTH AMERICA
- 10.2.1 MACROECONOMIC OUTLOOK
- 10.2.2 US
- 10.2.2.1 Growing cases of organ failure and underlying diseases to drive demand
- 10.2.3 CANADA
- 10.2.3.1 Improved organ donation rates to drive growth
- 10.3 EUROPE
- 10.3.1 MACROECONOMIC OUTLOOK
- 10.3.2 GERMANY
- 10.3.2.1 Growing patient population to drive demand
- 10.3.3 FRANCE
- 10.3.3.1 Increasing number of organ transplants and organ donor registrations to drive growth
- 10.3.4 UK
- 10.3.4.1 Increasing disease/disorder prevalence and favorable regulations to drive demand
- 10.3.5 SPAIN
- 10.3.5.1 High donation rates to drive growth
- 10.3.6 ITALY
- 10.3.6.1 Surge in donor numbers and technological innovation to drive growth
- 10.3.7 REST OF EUROPE
- 10.4 ASIA PACIFIC
- 10.4.1 MACROECONOMIC OUTLOOK
- 10.4.2 CHINA
- 10.4.2.1 Growing awareness and adoption of laws favoring organ donation to drive market
- 10.4.3 JAPAN
- 10.4.3.1 Nationwide awareness campaigns and promoted opt-in registration to drive growth
- 10.4.4 INDIA
- 10.4.4.1 Growing number of initiatives to increase public awareness to drive market
- 10.4.5 AUSTRALIA
- 10.4.5.1 Enhanced hospital-based donation programs to drive growth
- 10.4.6 SOUTH KOREA
- 10.4.6.1 Improved donor registration systems to drive growth
- 10.4.7 REST OF ASIA PACIFIC
- 10.5 LATIN AMERICA
- 10.5.1 MACROECONOMIC OUTLOOK
- 10.5.2 BRAZIL
- 10.5.2.1 Digitalization of donor registries to drive growth
- 10.5.3 MEXICO
- 10.5.3.1 Organ donation efforts supported by centralized transplant registry and national coordination to drive growth
- 10.5.4 REST OF LATIN AMERICA
- 10.6 MIDDLE EAST & AFRICA
- 10.6.1 IMPLEMENTATION OF GOVERNMENT INITIATIVES AND POLICIES TO DRIVE MARKET
- 10.6.2 MACROECONOMIC OUTLOOK
- 10.7 GCC COUNTRIES
- 10.7.1 STRONG ORGAN PRESERVATION THROUGH INCENTIVES, INFRASTRUCTURE, AND PUBLIC AWARENESS TO DRIVE GROWTH
- 10.7.2 MACROECONOMIC OUTLOOK
11 COMPETITIVE LANDSCAPE
- 11.1 OVERVIEW
- 11.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 11.3 REVENUE ANALYSIS, 2022-2024
- 11.4 MARKET SHARE ANALYSIS
- 11.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 11.5.1 STARS
- 11.5.2 EMERGING LEADERS
- 11.5.3 PERVASIVE PLAYERS
- 11.5.4 PARTICIPANTS
- 11.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 11.5.5.1 Company footprint
- 11.5.5.2 Solution footprint
- 11.5.5.3 Technique footprint
- 11.5.5.4 End user footprint
- 11.5.5.5 Region footprint
- 11.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 11.6.1 PROGRESSIVE COMPANIES
- 11.6.2 RESPONSIVE COMPANIES
- 11.6.3 DYNAMIC COMPANIES
- 11.6.4 STARTING BLOCKS
- 11.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 11.6.5.1 List of key startups/SMEs
- 11.6.5.2 Competitive benchmarking of key startups/SMEs
- 11.7 COMPANY VALUATION AND FINANCIAL METRICS
- 11.7.1 COMPANY VALUATION
- 11.7.2 FINANCIAL METRICS
- 11.8 BRAND/PRODUCT COMPARISON
- 11.9 COMPETITIVE SCENARIO
- 11.9.1 PRODUCT LAUNCHES & APPROVALS
- 11.9.2 DEALS
- 11.9.3 OTHER DEVELOPMENTS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- 12.1.1 PARAGONIX TECHNOLOGIES, INC.
- 12.1.1.1 Business overview
- 12.1.1.2 Products offered
- 12.1.1.3 Recent developments
- 12.1.1.3.1 Product launches
- 12.1.1.3.2 Deals
- 12.1.1.3.3 Other developments
- 12.1.1.4 MnM view
- 12.1.1.4.1 Right to win
- 12.1.1.4.2 Strategic choices
- 12.1.1.4.3 Weaknesses and competitive threats
- 12.1.2 XVIVO PERFUSION AB
- 12.1.2.1 Business overview
- 12.1.2.2 Products offered
- 12.1.2.3 Recent developments
- 12.1.2.3.1 Product launches & approvals
- 12.1.2.3.2 Deals
- 12.1.2.4 MnM view
- 12.1.2.4.1 Right to win
- 12.1.2.4.2 Strategic choices
- 12.1.2.4.3 Weaknesses and competitive threats
- 12.1.3 DR. FRANZ KOHLER CHEMIE GMBH
- 12.1.3.1 Business overview
- 12.1.3.2 Products offered
- 12.1.3.3 MnM view
- 12.1.3.3.1 Right to win
- 12.1.3.3.2 Strategic choices
- 12.1.3.3.3 Weaknesses and competitive threats
- 12.1.4 ESSENTIAL PHARMACEUTICALS, LLC (SUBSIDIARY OF ACCORD HEALTHCARE)
- 12.1.4.1 Business overview
- 12.1.4.2 Products offered
- 12.1.4.3 MnM view
- 12.1.4.3.1 Right to win
- 12.1.4.3.2 Strategic choices
- 12.1.4.3.3 Weaknesses and competitive threats
- 12.1.5 TRANSMEDICS, INC.
- 12.1.5.1 Business overview
- 12.1.5.2 Products offered
- 12.1.5.3 Recent developments
- 12.1.5.3.1 Product launches & approvals
- 12.1.5.3.2 Deals
- 12.1.5.4 MnM view
- 12.1.5.4.1 Right to win
- 12.1.5.4.2 Strategic choices
- 12.1.5.4.3 Weaknesses and competitive threats
- 12.1.6 ORGANOX LIMITED
- 12.1.6.1 Business overview
- 12.1.6.2 Products offered
- 12.1.6.3 Recent developments
- 12.1.6.3.1 Product launches & approvals
- 12.1.6.3.2 Deals
- 12.1.7 21ST CENTURY MEDICINE
- 12.1.7.1 Business overview
- 12.1.7.2 Products offered
- 12.1.8 BIOLIFE SOLUTIONS, INC.
- 12.1.8.1 Business overview
- 12.1.8.2 Products offered
- 12.1.8.3 Recent developments
- 12.1.8.3.1 Product launches
- 12.1.8.3.2 Deals
- 12.1.9 BRIDGE TO LIFE LIMITED
- 12.1.9.1 Business overview
- 12.1.9.2 Products offered
- 12.1.9.3 Recent developments
- 12.1.9.3.1 Product launches & approvals
- 12.1.9.3.2 Deals
- 12.1.10 WATERS MEDICAL SYSTEMS LLC
- 12.1.10.1 Business overview
- 12.1.10.2 Products offered
- 12.1.1 PARAGONIX TECHNOLOGIES, INC.
- 12.2 OTHER PLAYERS
- 12.2.1 SHANGHAI GENEXT MEDICAL TECHNOLOGY CO., LTD.
- 12.2.2 PRESERVATION SOLUTIONS, INC.
- 12.2.3 CARNAMEDICA
- 12.2.4 TRANSPLANT BIOMEDICALS
- 12.2.5 INSTITUT GEORGES LOPEZ
- 12.2.6 GLOBAL TRANSPLANT SOLUTIONS
- 12.2.7 AVIONORD
- 12.2.8 ORGAN PRESERVATION SOLUTIONS LTD.
- 12.2.9 EBERS
- 12.2.10 S.A.L.F.
- 12.2.11 BIOCHEFA
- 12.2.12 VASCULAR PERFUSION SOLUTIONS, INC.
- 12.2.13 TX INNOVATIONS
- 12.2.14 TAIYO NIPPON SANSO CORPORATION
- 12.2.15 X-THERMA, INC.
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGE STORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 AVAILABLE CUSTOMIZATIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS



















