
|
시장보고서
상품코드
1787261
바이오의약품 수탁 제조 시장 : 서비스별, 유형별, 사업 규모별, 원료별, 분자 유형별, 치료 영역별, 지역별 예측(-2030년)Biopharmaceutical Contract Manufacturing Market by Service, Type, Scale, Source, Therapy Area, Molecule Type - Global Forecast to 2030 |
||||||
세계의 바이오의약품 수탁 제조 시장 규모는 2025년 추정 224억 달러에서 2030년에는 341억 5,000만 달러에 이르고, 2025년부터 2030년까지 CAGR 8.8%를 보일 것으로 예측됩니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2030년 |
| 기준연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 검토 단위 | 금액(10억 달러) |
| 부문 | 서비스별, 유형별, 사업 규모별, 원료별, 분자유형별, 치료영역별, 지역별 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
바이오 의약품 수탁 제조 시장의 성장은 주로 제조 기술의 진보와 맞춤형 의료의 중요성 증가에 의해 발생합니다. 그러나 규제의 진화가 과제가 되어 시장 확대가 제한될 가능성도 있습니다.
2024년 바이오 의약품 수탁 제조 시장에서는 단일클론항체 분야가 가장 큰 점유율을 차지했습니다.
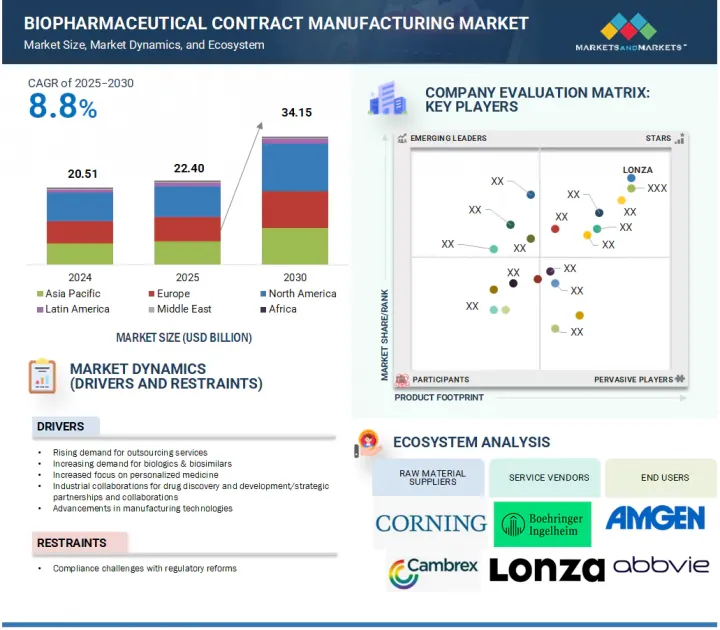
바이오의약품 수탁 제조 시장은 분자 유형별로 단일클론항체(mAbs), 세포 및 유전자 치료, 항체 약물 복합체, 백신, 치료용 펩티드 및 단백질, 기타 분자로 구분됩니다. 암이나 자가면역질환과 같은 만성질환의 유병률이 상승하고 표적 치료에 대한 수요가 높아지고 있기 때문에 mAbs 분야가 시장을 선도하고 있습니다. 단일클론항체는 특정 질병 경로를 표적으로 하는 정확도가 높기 때문에 임상 현장에서 선호됩니다. mAb 연구에 대한 정부의 지원은 바이오제약기업의 파이프라인 확충을 촉진하고 임상시험 중 mAb 후보의 수를 늘리고 있습니다. 그 결과, 제조 위탁 기관은 이러한 첨단 생물 제제를 효율적이고 비용 효율적으로 제조하는 서비스에 대한 수요가 증가하고 있습니다.
포유류 발현 시스템 부문은 주로 단일 클론 항체와 같은 복잡한 생물학적 제제의 제조에 중요한 역할을 하기 때문에 세계 바이오의약품 수탁 제조 시장을 독점하고 있습니다. 이 이점은 포유류 세포 플랫폼이 복잡한 번역 후 변형을 수행하고 치료 효과를 향상시킬 수 있기 때문입니다. 첨단 생물학적 제제에 대한 수요가 증가하고 세포 배양 기술에 대한 투자가 증가함에 따라 이 부문이 더욱 견고해집니다. 제조 위탁 기관은 포유류 세포 배양 능력을 확대하고 수율과 품질을 향상시키기 위해 첨단 기술을 채택하고 있습니다. 생물 제제의 파이프라인이 성장함에 따라 포유류 발현 시스템 부문은 바이오 의약품 제조의 성장과 기술 혁신을 계속 견인할 것으로 예측됩니다.
2025년부터 2030년까지 시장 점유율은 유럽이 2위를 차지할 것으로 예상됩니다.
유럽의 바이오의약품 수탁제조 시장은 단일클론항체나 세포 및 유전자 치료 등 생물제제에 대한 투자 증가와 바이오제조능력 확대가 견인해 예측기간 중에는 2위 시장 규모를 자랑합니다. 이 지역은 혁신의 중심지이며 품질과 효율성을 높이는 첨단 기술에 주력하고 있습니다. 예를 들어, 2023년 3월에는 Lonza가 대규모 의약품 제조를 위한 cGMP 제조 사이트를 시작하여 복잡한 생물제제의 세계적 수요에 부응하는 유럽의 헌신을 명확히 하고, 최상급의 위탁제조 서비스를 요구하는 제약 및 바이오 기업을 끌고 있습니다.
본 보고서에서는 세계의 바이오의약품 수탁 제조 시장에 대해 조사했으며, 서비스별, 유형별, 사업 규모별, 원료별, 분자유형별, 치료영역별, 지역별 동향 및 시장 진출기업 프로파일 등을 정리했습니다.
목차
제1장 서론
제2장 조사 방법
제3장 주요 요약
제4장 중요 인사이트
제5장 시장 개요
- 소개
- 시장 역학
- 기술 분석
- 고객의 비즈니스에 영향을 미치는 동향/혼란
- 가격 분석(정성)
- 밸류체인 분석
- 생태계 분석
- 특허 분석
- 규제 분석
- 2025-2026년의 주된 회의와 이벤트
- Porter's Five Forces 분석
- 주요 이해관계자와 구매 기준
- 투자 및 자금조달 시나리오
- AI/생성형 AI가 바이오의약품 수탁 제조 시장에 미치는 영향
- 2025년 미국 관세가 바이오의약품 수탁 제조 시장에 미치는 영향
제6장 바이오의약품 수탁 제조 시장(서비스별)
- 소개
- 제조
- 배합과 충전 및 마무리
- 포장과 라벨
- 기타
제7장 바이오 의약품 수탁 제조 시장(유형별)
- 소개
- 생물학적 제제 원약 제조
- 생물학적 제제 제조
제8장 바이오의약품 수탁 제조 시장(사업 규모별)
- 소개
- 상업사업
- 임상 업무
제9장 바이오의약품 수탁 제조 시장(원료별)
- 소개
- 포유류 발현계
- 비포유류 발현계
제10장 바이오의약품 수탁 제조 시장(분자 유형별)
- 소개
- 단일클론항체
- 세포 및 유전자 치료
- 항체 약물 복합체
- 백신
- 치료용 펩티드와 단백질
- 기타
제11장 바이오의약품 수탁 제조 시장(치료 영역별)
- 소개
- 종양학
- 자가면역질환
- 심혈관 질환
- 대사성 질환
- 감염증
- 신경학
- 기타
제12장 바이오의약품 수탁 제조 시장(지역별)
- 소개
- 북미
- 북미의 거시경제 전망
- 미국
- 캐나다
- 유럽
- 유럽의 거시 경제 전망
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 스위스
- 아일랜드
- 기타
- 아시아태평양
- 아시아태평양의 거시 경제 전망
- 중국
- 일본
- 인도
- 한국
- 기타
- 라틴아메리카
- 라틴아메리카의 거시 경제 전망
- 브라질
- 멕시코
- 기타
- 중동
- 중동의 거시 경제 전망
- GCC 국가
- 기타
- 아프리카
- 시장 확대를 위한 현지 의약품 생산 개선의 대처
- 아프리카의 거시 경제 전망
제13장 경쟁 구도
- 개요
- 바이오의약품 수탁 제조 시장에 있어서의 주요 기업이 채용하는 전략의 개요(2022-2025년)
- 수익 분석, 2020-2024년
- 시장 점유율 분석, 2024년
- 기업평가 매트릭스 : 주요 진입기업, 2024년
- 기업평가 매트릭스 : 중소기업/스타트업, 2024년
- 기업평가와 재무지표
- 브랜드/제품 비교
- 경쟁 시나리오
제14장 기업 프로파일
- 주요 진출기업
- LONZA
- WUXI BIOLOGICS
- SAMSUNG BIOLOGICS
- THERMO FISHER SCIENTIFIC INC.
- ABBVIE INC.
- CATALENT, INC.
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH
- FUJIFILM HOLDINGS CORPORATION
- EUROFINS SCIENTIFIC
- GENSCRIPT BIOTECH CORPORATION
- AGC INC.
- MERCK KGAA
- JSR CORPORATION
- IDT BIOLOGIKA
- AJINOMOTO BIO-PHARMA
- AGILENT TECHNOLOGIES INC.
- ASAHI KASEI CORPORATION
- 기타 기업
- ONESOURCE PHARMA SOLUTIONS
- RECIPHARM AB
- EMERGENT BIOSOLUTIONS
- SHANGHAI FOSUN PHARMACEUTICAL CO., LTD.
- LOTTE BIOLOGICS
- CURIA GLOBAL, INC.
- POLYPLUS TRANSFECTION
- ALDEVRON LLC
- MINAPHARM PHARMACEUTICALS
- RENTSCHLER BIOPHARMA SE
- PORTON PHARMA SOLUTIONS
- CELLARES, INC.
- MABPLEX INTERNATIONAL CO., LTD.
- ASYMCHEM INC.
제15장 부록
JHS 25.08.14The global biopharmaceutical contract manufacturing market is expected to reach USD 34.15 billion by 2030 from an estimated USD 22.40 billion in 2025, at a CAGR of 8.8% from 2025 to 2030.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Service, Type, Source, Scale of Operation, Molecule Type, Therapeutic Area, and Region |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa |
This growth in the biopharmaceutical contract manufacturing market is primarily driven by advancements in manufacturing technologies and a growing emphasis on personalized medicine. However, evolving regulations may pose challenges that could limit market expansion.
The monoclonal antibodies segment accounted for the largest share of the biopharmaceutical contract manufacturing market in 2024.
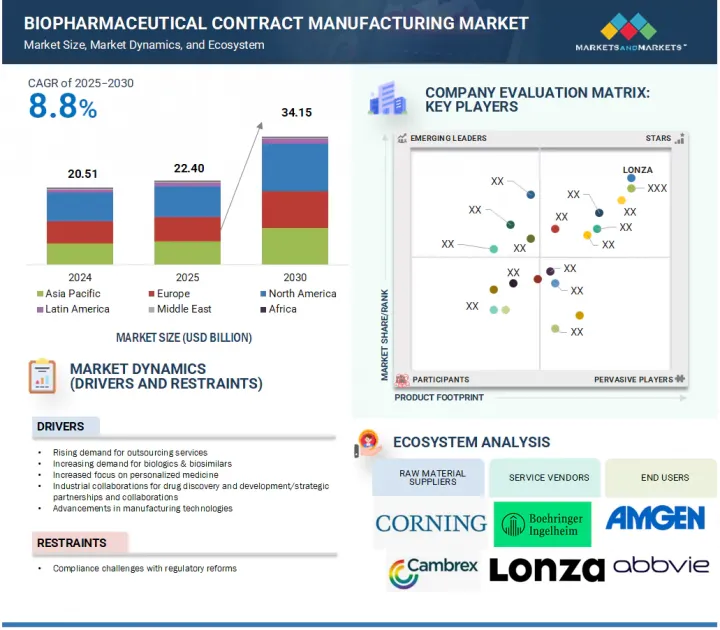
The biopharmaceutical contract manufacturing market is segmented by molecule type into monoclonal antibodies (mAbs), cell & gene therapy, antibody-drug conjugates, vaccines, therapeutic peptides & proteins, and other molecules. The mAbs segment leads the market due to the rising prevalence of chronic disorders like cancer and autoimmune diseases, driving the demand for targeted therapies. Monoclonal antibodies are preferred in clinical practice for their precision in targeting specific disease pathways. Government support for mAb research has encouraged biopharmaceutical firms to expand their pipelines, increasing the number of mAb candidates in clinical trials. Consequently, contract manufacturing organizations are experiencing a growing demand for their services to produce these advanced biologics efficiently and cost-effectively.
The mammalian expression systems segment dominated the market in 2024.
The mammalian expression systems segment dominates the global biopharmaceutical contract manufacturing market, primarily due to its critical role in creating complex biologics like monoclonal antibodies. This dominance stems from the ability of mammalian cell platforms to perform intricate post-translational modifications, enhancing treatment effectiveness. The rising demand for advanced biologics and increased investments in cell culture technologies have further solidified this segment. Contract manufacturing organizations are expanding their mammalian cell culture capabilities and adopting advanced technologies to improve yield and quality. As the pipeline for biologics grows, the mammalian expression systems segment is expected to continue driving growth and innovation in biopharmaceutical manufacturing.
Europe accounted for the second-largest share of the market from 2025 to 2030.
The biopharmaceutical contract manufacturing market in Europe is the second largest during the forecast period, driven by rising investments in biologics such as monoclonal antibodies and cell & gene therapies, along with expansions in biomanufacturing capacity. The region is a hub for innovation, focusing on advanced technologies that enhance quality and efficiency. For example, in March 2023, Lonza launched a cGMP manufacturing site for large-scale drug production, underscoring Europe's commitment to meeting the global demand for complex biologics and attracting pharmaceutical and biotech firms seeking top-tier contract manufacturing services.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side (70%) and Demand Side (30%)
- By Designation: Managers (45%), CXOs and Directors (30%), and Executives (25%)
- By Region: North America (40%), Europe (25%), the Asia Pacific (25%), Latin America (5%), and the Middle East & Africa (5%)
List of Key Companies Profiled in the Report:
Key players in the biopharmaceutical contract manufacturing market include Lonza (Switzerland), Thermo Fisher Scientific Inc. (US), WuXi Biologics (China), Catalent, Inc. (US), Samsung Biologics (South Korea), Boehringer Ingelheim International GmbH (Germany), FUJIFILM Holdings Corporation (Japan), AbbVie, Inc. (US), Eurofins Scientific (Luxembourg), GenScript Biotech Corporation (US), AGC Inc. (Japan), Merck KGaA (Germany), JSR Corporation (Japan), IDT Biologika (Germany), Ajinomoto Bio-Pharma (Japan), Agilent Technologies, Inc. (US), and Asahi Kasei Corporation (Japan).
Research Coverage:
This research report categorizes the biopharmaceutical contract manufacturing market by service (manufacturing, formulation & fill-finish, packaging & labeling, and other services), type (biologic drug substance manufacturing and biologic drug product manufacturing), scale of operation (clinical and commercial operations), source (mammalian and non-mammalian expression systems), therapeutic area (oncology, autoimmune diseases, metabolic diseases, cardiovascular diseases, neurology, infectious diseases, and other therapeutic areas), molecule type (monoclonal antibodies, antibody-drug conjugates, cell & gene therapy, vaccines, therapeutic peptides & proteins, and other molecules), and region (North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa).
The report provides a comprehensive analysis of the key factors affecting the growth of the biopharmaceutical contract manufacturing market, including drivers, restraints, challenges, and opportunities. It includes an in-depth examination of major industry players, offering insights into their business overviews, products, solutions, and key strategies, as well as their collaborations, partnerships, and agreements. Additionally, the report highlights recent developments related to new approvals and launches, mergers and acquisitions, and other significant activities in the biopharmaceutical contract manufacturing market.
Key Benefits of Buying the Report:
The report will assist both market leaders and new entrants by providing accurate revenue estimates for the overall biopharmaceutical contract manufacturing market and its subsegments. It will also help stakeholders better understand the competitive landscape, enabling them to better position their businesses and develop effective go-to-market strategies. Additionally, this report will offer insights into the market's dynamics, including key drivers, constraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (rising demand for outsourcing services, increasing demand for biologics & biosimilars, increased focus on personalized medicine, industrial collaborations for drug discovery and development/strategic partnerships and collaborations, and advancements in manufacturing technologies), restraints (challenges complying with regulatory reforms), opportunities (rising demand for cell & gene therapy, emerging countries market, and strong emphasis on drug development), and challenges (challenges to meet reformed regulations) influencing the growth of the market.
- Service Development/Innovation: Detailed insights on the upcoming technologies and research & development activities in the biopharmaceutical contract manufacturing market.
- Market Development: The report provides comprehensive information about profitable markets by analyzing them across various regions.
- Market Diversification: Exhaustive information about untapped geographies, recent developments, and investments in the biopharmaceutical contract manufacturing market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players. A detailed analysis of the key industry players has been done to provide insights into their key strategies, service launches/approvals, pipeline analysis, acquisitions, partnerships, agreements, collaborations, other recent developments, investment and funding activities, brand/product comparative analysis, and vendor valuation and financial metrics of the biopharmaceutical contract manufacturing market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Objectives of secondary research
- 2.1.1.2 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Breakdown of primaries (supply- and demand-side participants)
- 2.1.2.2 Key objective of primary research
- 2.1.1 SECONDARY DATA
- 2.2 MARKET ESTIMATION
- 2.2.1 GLOBAL MARKET ESTIMATION
- 2.2.1.1 Company revenue analysis (bottom-up approach)
- 2.2.1.1.1 Revenue share analysis of Thermo Fisher Scientific Inc.
- 2.2.1.2 MnM repository analysis
- 2.2.1.3 Primary interviews
- 2.2.1.1 Company revenue analysis (bottom-up approach)
- 2.2.2 INSIGHTS OF PRIMARY EXPERTS
- 2.2.3 SEGMENTAL MARKET SIZE ESTIMATION (TOP-DOWN APPROACH)
- 2.2.1 GLOBAL MARKET ESTIMATION
- 2.3 MARKET GROWTH RATE PROJECTIONS
- 2.4 DATA TRIANGULATION
- 2.5 STUDY ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET OVERVIEW
- 4.2 NORTH AMERICA: BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SCALE OF OPERATION AND COUNTRY (2025)
- 4.3 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET SHARE, BY THERAPEUTIC AREA, 2025 VS. 2030
- 4.4 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE TYPE, 2025 VS. 2030 (USD MILLION)
- 4.5 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Increasing outsourcing of biologics manufacturing among biopharmaceutical companies
- 5.2.1.2 Rising demand for biologics and biosimilars
- 5.2.1.3 Growing focus on personalized medicine
- 5.2.1.4 Increasing collaborations between pharmaceutical companies and biologics CMOs
- 5.2.1.5 Advancements in manufacturing technologies
- 5.2.2 RESTRAINTS
- 5.2.2.1 Intellectual property rights issues
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Rising demand for cell & gene therapy
- 5.2.3.2 Significant growth opportunities offered by emerging countries
- 5.2.3.3 Expansion of biologics manufacturing capacities by CMOs
- 5.2.4 CHALLENGES
- 5.2.4.1 Strict regulations
- 5.2.1 DRIVERS
- 5.3 TECHNOLOGY ANALYSIS
- 5.3.1 KEY TECHNOLOGIES
- 5.3.1.1 Single-use technologies
- 5.3.1.2 Continuous bioprocessing
- 5.3.2 COMPLEMENTARY TECHNOLOGIES
- 5.3.2.1 Robotics and automation in fill-finish
- 5.3.2.2 CRISPR & gene editing tools
- 5.3.3 ADJACENT TECHNOLOGIES
- 5.3.3.1 3D bioprinting & microfluidics
- 5.3.3.2 Biosensors & real-time analytics
- 5.3.1 KEY TECHNOLOGIES
- 5.4 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- 5.5 PRICING ANALYSIS (QUALITATIVE)
- 5.6 VALUE CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- 5.8 PATENT ANALYSIS
- 5.9 REGULATORY ANALYSIS
- 5.9.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.9.2 REGULATORY LANDSCAPE
- 5.10 KEY CONFERENCES & EVENTS, 2025-2026
- 5.11 PORTER'S FIVE FORCES ANALYSIS
- 5.11.1 THREAT OF NEW ENTRANTS
- 5.11.2 THREAT OF SUBSTITUTES
- 5.11.3 BARGAINING POWER OF SUPPLIERS
- 5.11.4 BARGAINING POWER OF BUYERS
- 5.11.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.12 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.12.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.12.2 BUYING CRITERIA
- 5.13 INVESTMENT & FUNDING SCENARIO
- 5.14 IMPACT OF AI/GEN AI ON BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET
- 5.15 IMPACT OF 2025 US TARIFFS ON BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET
- 5.15.1 INTRODUCTION
- 5.15.2 KEY TARIFF RATES
- 5.15.3 PRICE IMPACT ANALYSIS
- 5.15.4 IMPACT ON COUNTRY/REGION
- 5.15.4.1 US
- 5.15.4.2 Europe
- 5.15.4.3 APAC
- 5.15.5 IMPACT ON UPSTREAM INDUSTRIES
6 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE
- 6.1 INTRODUCTION
- 6.2 MANUFACTURING
- 6.2.1 SHIFT TOWARD CONTINUOUSLY IMPROVING SINGLE-USE TECHNOLOGIES TO DRIVE MARKET
- 6.3 FORMULATION & FILL-FINISH
- 6.3.1 CRITICAL ROLE OF FORMULATION & FILL-FINISH IN BIOLOGICS MANUFACTURING TO DRIVE MARKET GROWTH
- 6.4 PACKAGING & LABELING
- 6.4.1 IMPORTANCE OF PACKAGING & LABELING IN ENSURING SAFETY AND EFFECTIVENESS TO SUPPORT MARKET GROWTH
- 6.5 OTHER SERVICES
7 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY TYPE
- 7.1 INTRODUCTION
- 7.2 BIOLOGIC DRUG SUBSTANCE MANUFACTURING
- 7.2.1 INCREASING DEMAND FOR BIOTECHNOLOGY PRODUCTS TO DRIVE MARKET
- 7.3 BIOLOGIC DRUG PRODUCT MANUFACTURING
- 7.3.1 INCREASING R&D COST AND PROCESS COMPLEXITY TO DRIVE DEMAND FOR CONTRACT MANUFACTURING FOR BIOLOGIC DRUG PRODUCTS
8 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SCALE OF OPERATION
- 8.1 INTRODUCTION
- 8.2 COMMERCIAL OPERATIONS
- 8.2.1 INCREASING APPROVALS OF BIOLOGICS TO SUPPORT MARKET GROWTH
- 8.3 CLINICAL OPERATIONS
- 8.3.1 INCREASING NUMBER OF CLINICAL TRIALS FOR TARGETED THERAPIES TO DRIVE MARKET
9 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SOURCE
- 9.1 INTRODUCTION
- 9.2 MAMMALIAN EXPRESSION SYSTEMS
- 9.2.1 VERSATILE THERAPEUTIC DIVERSITY OF MAMMALIAN EXPRESSION SYSTEMS TO DRIVE MARKET
- 9.3 NON-MAMMALIAN EXPRESSION SYSTEMS
- 9.3.1 INCREASING DEMAND FOR BIOLOGICS VACCINES TO SUPPORT MARKET GROWTH
10 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE TYPE
- 10.1 INTRODUCTION
- 10.2 MONOCLONAL ANTIBODIES
- 10.2.1 GROWING APPROVALS FOR MABS TO PROPEL MARKET
- 10.3 CELL & GENE THERAPY
- 10.3.1 INCREASING PREVALENCE OF CHRONIC DISEASES TO DRIVE MARKET
- 10.4 ANTIBODY-DRUG CONJUGATES
- 10.4.1 INCREASING INVESTMENTS IN BIOLOGICS TO SUPPORT MARKET GROWTH
- 10.5 VACCINES
- 10.5.1 INCREASING DEMAND FOR VACCINES TO DRIVE MARKET
- 10.6 THERAPEUTIC PEPTIDES & PROTEINS
- 10.6.1 INCREASING NUMBER OF RESEARCH PROJECTS IN GENOMICS TO SUPPORT MARKET GROWTH
- 10.7 OTHER MOLECULES
11 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY THERAPEUTIC AREA
- 11.1 INTRODUCTION
- 11.2 ONCOLOGY
- 11.2.1 INCREASED APPROVALS AND LAUNCH OF BIOLOGICS FOR ONCOLOGY TO DRIVE MARKET
- 11.3 AUTOIMMUNE DISEASES
- 11.3.1 RISING PREVALENCE OF AUTOIMMUNE DISEASES TO DRIVE MARKET
- 11.4 CARDIOVASCULAR DISEASES
- 11.4.1 DEVELOPMENT OF NEW TREATMENT OPTIONS TO SUPPORT MARKET GROWTH
- 11.5 METABOLIC DISEASES
- 11.5.1 INCREASING PREVALENCE OF METABOLIC DISEASES TO DRIVE ADOPTION OF BIOLOGICS
- 11.6 INFECTIOUS DISEASES
- 11.6.1 RISING EPIDEMIC OUTBREAKS TO SUPPORT MARKET GROWTH
- 11.7 NEUROLOGY
- 11.7.1 HIGH BURDEN OF NEUROLOGICAL DISORDERS TO PROPEL MARKET
- 11.8 OTHER THERAPEUTIC AREAS
12 BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY REGION
- 12.1 INTRODUCTION
- 12.2 NORTH AMERICA
- 12.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 12.2.2 US
- 12.2.2.1 Presence of large number of FDA-approved manufacturing facilities to favor market growth
- 12.2.3 CANADA
- 12.2.3.1 Rising government funding for drug development to support market growth
- 12.3 EUROPE
- 12.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 12.3.2 GERMANY
- 12.3.2.1 Rapidly growing pharmaceutical market to drive market
- 12.3.3 UK
- 12.3.3.1 Rising investments in drug development to favor market growth
- 12.3.4 FRANCE
- 12.3.4.1 Availability of funds from government and private organizations for domestic drug development to support market growth
- 12.3.5 ITALY
- 12.3.5.1 Rising commercial drug development pipeline to drive market
- 12.3.6 SPAIN
- 12.3.6.1 Rising R&D expenditure to propel market
- 12.3.7 SWITZERLAND
- 12.3.7.1 Strong R&D investments and infrastructure expansion to bolster biotech manufacturing
- 12.3.8 IRELAND
- 12.3.8.1 Robust R&D growth and CDMO capacity expansion to fuel pharma manufacturing
- 12.3.9 REST OF EUROPE
- 12.4 ASIA PACIFIC
- 12.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 12.4.2 CHINA
- 12.4.2.1 Low manufacturing cost and high demand for medicines to favor market growth
- 12.4.3 JAPAN
- 12.4.3.1 Growing demand for biosimilars to drive growth
- 12.4.4 INDIA
- 12.4.4.1 Increasing pharma R&D activities and government funding for biotechnology industry to support market growth
- 12.4.5 SOUTH KOREA
- 12.4.5.1 Increasing R&D activities for drug development to drive market
- 12.4.6 REST OF ASIA PACIFIC
- 12.5 LATIN AMERICA
- 12.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 12.5.2 BRAZIL
- 12.5.2.1 Growing pharmaceutical industry to drive market
- 12.5.3 MEXICO
- 12.5.3.1 Regulatory Improvements to support market growth
- 12.5.4 REST OF LATIN AMERICA
- 12.6 MIDDLE EAST
- 12.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST
- 12.6.2 GCC COUNTRIES
- 12.6.2.1 Substantial opportunities fueled by strong government support to boost growth
- 12.6.3 REST OF MIDDLE EAST
- 12.7 AFRICA
- 12.7.1 EFFORTS TO IMPROVE LOCAL PHARMACEUTICAL PRODUCTION TO BOOST MARKET
- 12.7.2 MACROECONOMIC OUTLOOK FOR AFRICA
13 COMPETITIVE LANDSCAPE
- 13.1 OVERVIEW
- 13.2 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN BIOPHARMACEUTICAL CONTRACT MANUFACTURING MARKET, 2022-2025
- 13.3 REVENUE ANALYSIS, 2020-2024
- 13.4 MARKET SHARE ANALYSIS, 2024
- 13.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 13.5.1 STARS
- 13.5.2 EMERGING LEADERS
- 13.5.3 PERVASIVE PLAYERS
- 13.5.4 PARTICIPANTS
- 13.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 13.5.5.1 Company footprint
- 13.5.5.2 Region footprint
- 13.5.5.3 Service type footprint
- 13.5.5.4 Molecule type footprint
- 13.5.5.5 Therapeutic area footprint
- 13.6 COMPANY EVALUATION MATRIX: SMES/STARTUPS, 2024
- 13.6.1 PROGRESSIVE COMPANIES
- 13.6.2 RESPONSIVE COMPANIES
- 13.6.3 DYNAMIC COMPANIES
- 13.6.4 STARTING BLOCKS
- 13.6.5 COMPETITIVE BENCHMARKING: SMES/STARTUPS, 2024
- 13.6.5.1 Detailed list of key SMEs/startups
- 13.6.5.2 Competitive benchmarking of key emerging players/startups
- 13.7 COMPANY VALUATION & FINANCIAL METRICS
- 13.7.1 FINANCIAL METRICS
- 13.7.2 COMPANY VALUATION
- 13.8 BRAND/PRODUCT COMPARISON
- 13.9 COMPETITIVE SCENARIO
- 13.9.1 SERVICE LAUNCHES
- 13.9.2 DEALS
- 13.9.3 EXPANSIONS
14 COMPANY PROFILES
- 14.1 KEY PLAYERS
- 14.1.1 LONZA
- 14.1.1.1 Business overview
- 14.1.1.2 Services offered
- 14.1.1.3 Recent developments
- 14.1.1.3.1 Deals
- 14.1.1.3.2 Expansions
- 14.1.1.4 MnM view
- 14.1.1.4.1 Key strengths
- 14.1.1.4.2 Strategic choices
- 14.1.1.4.3 Weaknesses & competitive threats
- 14.1.2 WUXI BIOLOGICS
- 14.1.2.1 Business overview
- 14.1.2.2 Services offered
- 14.1.2.3 Recent developments
- 14.1.2.3.1 Deals
- 14.1.2.3.2 Expansions
- 14.1.2.4 MnM view
- 14.1.2.4.1 Key strengths
- 14.1.2.4.2 Strategic choices
- 14.1.2.4.3 Weaknesses & competitive threats
- 14.1.3 SAMSUNG BIOLOGICS
- 14.1.3.1 Business overview
- 14.1.3.2 Services offered
- 14.1.3.3 Recent developments
- 14.1.3.3.1 Service launches
- 14.1.3.3.2 Deals
- 14.1.3.3.3 Expansions
- 14.1.3.4 MnM view
- 14.1.3.4.1 Key strengths
- 14.1.3.4.2 Strategic choices
- 14.1.3.4.3 Weaknesses & competitive threats
- 14.1.4 THERMO FISHER SCIENTIFIC INC.
- 14.1.4.1 Business overview
- 14.1.4.2 Services offered
- 14.1.4.3 Recent developments
- 14.1.4.3.1 Deals
- 14.1.4.4 MnM view
- 14.1.4.4.1 Key strengths
- 14.1.4.4.2 Strategic choices
- 14.1.4.4.3 Weaknesses & competitive threats
- 14.1.5 ABBVIE INC.
- 14.1.5.1 Business overview
- 14.1.5.2 Services offered
- 14.1.6 CATALENT, INC.
- 14.1.6.1 Business overview
- 14.1.6.2 Services offered
- 14.1.6.3 Recent developments
- 14.1.6.3.1 Deals
- 14.1.6.3.2 Expansions
- 14.1.6.4 MnM view
- 14.1.6.4.1 Key strengths
- 14.1.6.4.2 Strategic choices
- 14.1.6.4.3 Weaknesses & competitive threats
- 14.1.7 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
- 14.1.7.1 Business overview
- 14.1.7.2 Services offered
- 14.1.7.3 Recent developments
- 14.1.7.3.1 Other developments
- 14.1.8 FUJIFILM HOLDINGS CORPORATION
- 14.1.8.1 Business overview
- 14.1.8.2 Services offered
- 14.1.8.3 Recent developments
- 14.1.8.3.1 Deals
- 14.1.8.3.2 Expansions
- 14.1.9 EUROFINS SCIENTIFIC
- 14.1.9.1 Business overview
- 14.1.9.2 Services offered
- 14.1.9.3 Recent developments
- 14.1.9.3.1 Expansions
- 14.1.10 GENSCRIPT BIOTECH CORPORATION
- 14.1.10.1 Business overview
- 14.1.10.2 Services offered
- 14.1.10.3 Recent developments
- 14.1.10.3.1 Service launches
- 14.1.10.3.2 Deals
- 14.1.10.3.3 Expansions
- 14.1.11 AGC INC.
- 14.1.11.1 Business overview
- 14.1.11.2 Services offered
- 14.1.11.3 Recent developments
- 14.1.11.3.1 Deals
- 14.1.11.3.2 Expansions
- 14.1.12 MERCK KGAA
- 14.1.12.1 Business overview
- 14.1.12.2 Services offered
- 14.1.12.3 Recent developments
- 14.1.12.3.1 Deals
- 14.1.12.3.2 Expansions
- 14.1.13 JSR CORPORATION
- 14.1.13.1 Business overview
- 14.1.13.2 Services offered
- 14.1.13.3 Recent developments
- 14.1.13.3.1 Expansions
- 14.1.14 IDT BIOLOGIKA
- 14.1.14.1 Business overview
- 14.1.14.2 Services offered
- 14.1.14.3 Recent developments
- 14.1.14.3.1 Other developments
- 14.1.15 AJINOMOTO BIO-PHARMA
- 14.1.15.1 Business overview
- 14.1.15.2 Services offered
- 14.1.15.3 Recent developments
- 14.1.15.3.1 Deals
- 14.1.16 AGILENT TECHNOLOGIES INC.
- 14.1.16.1 Business overview
- 14.1.16.2 Services offered
- 14.1.16.3 Recent developments
- 14.1.16.3.1 Deals
- 14.1.17 ASAHI KASEI CORPORATION
- 14.1.17.1 Business overview
- 14.1.17.2 Services offered
- 14.1.17.3 Recent developments
- 14.1.17.3.1 Deals
- 14.1.1 LONZA
- 14.2 OTHER PLAYERS
- 14.2.1 ONESOURCE PHARMA SOLUTIONS
- 14.2.2 RECIPHARM AB
- 14.2.3 EMERGENT BIOSOLUTIONS
- 14.2.4 SHANGHAI FOSUN PHARMACEUTICAL CO., LTD.
- 14.2.5 LOTTE BIOLOGICS
- 14.2.6 CURIA GLOBAL, INC.
- 14.2.7 POLYPLUS TRANSFECTION
- 14.2.8 ALDEVRON LLC
- 14.2.9 MINAPHARM PHARMACEUTICALS
- 14.2.10 RENTSCHLER BIOPHARMA SE
- 14.2.11 PORTON PHARMA SOLUTIONS
- 14.2.12 CELLARES, INC.
- 14.2.13 MABPLEX INTERNATIONAL CO., LTD.
- 14.2.14 ASYMCHEM INC.
15 APPENDIX
- 15.1 DISCUSSION GUIDE
- 15.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 15.3 CUSTOMIZATION OPTIONS
- 15.4 RELATED REPORTS
- 15.5 AUTHOR DETAILS















