
|
시장보고서
상품코드
1819091
면역조직화학 시장 : 제공별, 용도별, 최종사용자별, 지역별 - 예측(-2030년)Immunohistochemistry Market by Product & Service (Antibodies (Type (Primary), Clonality (Monoclonal), Regulatory Class, Reagents, Kits, Instruments (Stainers), Software)), Application (Diagnostics (Cancer), Research, Forensics) - Global Forecast to 2030 |
||||||
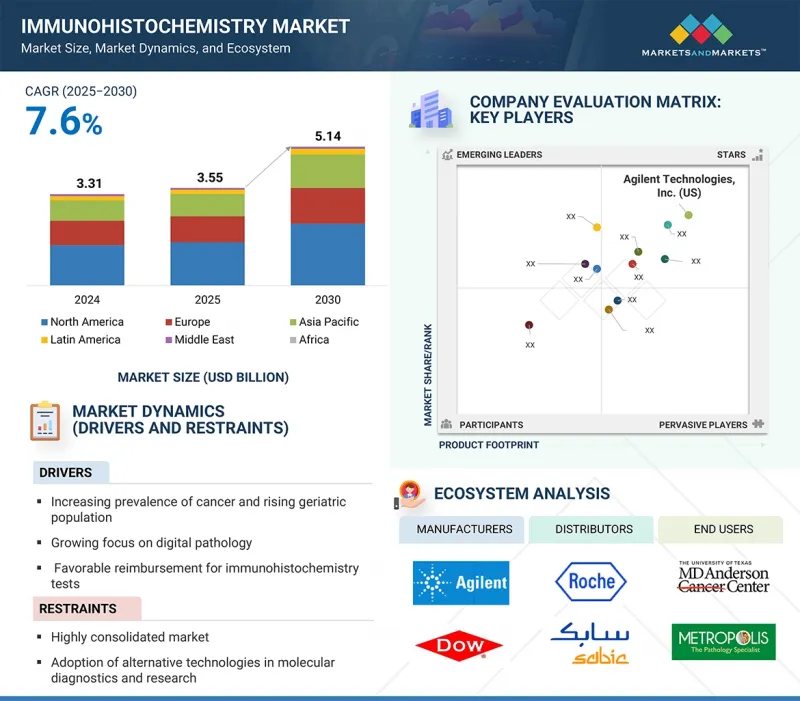
면역조직화학 시장 규모는 예측 기간 동안 7.6%의 CAGR로 확대되어 2024년 35억 5,000만 달러에서 2030년에는 51억 4,000만 달러에 달할 것으로 예측됩니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2030년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 검토 단위 | 금액(10억 달러) |
| 부문 | 제공별, 용도별, 최종사용자별, 지역별 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
시장 성장을 촉진하는 요인은 증가하는 노인 인구의 암 환자 유병률 증가와 암 진단 및 관리를 위한 IHC 검사의 적용 증가입니다. 또한, 동반진단약(CDx)의 개발과 맞춤형 의료를 개발하기 위한 IHC 기술과의 통합은 시장 확대를 더욱 촉진하고 있습니다.
시장은 조직 염색, 차단 혈청 및 시약, 발색 기질, 고정 시약, 희석제, 유기 용매, 단백질 분해 효소, 기타 시약으로 구분됩니다. 2024년, 조직 염색 부문은 면역조직화학 시장에서 가장 큰 점유율을 차지했습니다. 이 강력한 기술은 항원-항체 상호작용을 이용하여 조직 샘플 내의 특정 세포 성분, 단백질, 구조를 시각화하고 구별하기 위해 염색을 사용하는 기술입니다. 이를 통해 진단 및 연구에 필수적인 바이오마커의 정확한 국소화를 가능하게 합니다. 또한, 조직학적 염색의 유용성은 다중 염색(multiple staining)으로도 알려진 첨단 기술을 통해 확대되고 있습니다. 이 방법은 하나의 조직 절편 내에서 여러 표적을 검출하기 위해 여러 개의 염색을 동시에 사용하는 방법입니다. 이는 복잡한 종양 미세환경에 대한 종합적인 뷰를 제공하면서 귀중한 환자 샘플을 보존할 수 있기 때문에 면역종양학과 같은 분야에서 특히 유용합니다. 이 시장의 주요 촉진요인은 암 진단에 있어 매우 중요한 기능이며, 병리학자가 이러한 염색을 사용하여 정상 조직과 비정상 조직의 형태를 구별하고 이를 통해 종양의 악성도 및 종류를 판단하는 것입니다.
면역조직화학 시장은 진단용, 연구용, 법의학용으로 구분됩니다. 2024년에는 연구용 응용 분야가 면역조직화학 시장에서 두 번째 점유율을 차지했습니다. IHC 기술은 특히 항종양제 개발, 바이오마커 탐색 등 현대 생물의학 연구의 핵심입니다. IHC 기술은 연구자들에게 조직의 구조적 맥락에서 특정 단백질의 발현과 정확한 세포 내 위치를 시각화할 수 있는 독보적인 능력을 제공합니다. 이는 암 및 기타 질병을 뒷받침하는 복잡한 세포 메커니즘을 이해하는 데 기본이 됩니다. 예를 들어, 암 단백질체 탐색에서 IHC는 유전체 및 단백질체학 스크리닝을 통해 확인된 새로운 신약 타겟을 검증하고, 실제 종양 조직에서 그 존재와 연관성을 확인하는 데 도움을 주고 있습니다. 연구에서의 IHC의 역할은 많은 과학 분야에 걸쳐 있습니다. 발생생물학 및 발생학 연구에서는 발생의 다양한 단계에서 주요 조절 단백질을 매핑하여 형태 형성 및 선천성 질환을 이해하는 데 필수적인 인사이트를 제공합니다. 마찬가지로 줄기세포 연구에서도 IHC는 증식 및 분화 마커를 모니터링하는 데 사용되어 줄기세포의 거동과 치료 가능성에 대한 중요한 데이터를 제공합니다. 제약회사와 생명공학 기업들의 지속적인 연구개발 투자와 맞춤형 의료에 대한 전 세계적인 지원과 함께 연구 응용 분야가 면역조직화학 시장의 역동적이고 확장적인 요소로 자리매김할 수 있도록 보장합니다.
2024년 북미는 면역조직화학 시장에서 가장 큰 점유율을 차지했습니다. 이러한 선도적 지위는 이 지역의 고도로 발달된 의료 인프라, 막대한 의료비, 주요 시장 진입 기업 및 주요 연구기관의 견고한 존재 등 다양한 요인에 의해 뒷받침되고 있습니다. 특히 미국은 암을 필두로 한 만성질환의 높은 유병률에 힘입어 이러한 성장의 주요 원동력이 되고 있습니다. 다양한 암의 발병률이 크게 증가함에 따라 고도의 정확한 진단 도구가 필요하며, IHC는 종양 분류 및 바이오마커 분석을 위한 현대 병리학의 핵심이 되고 있습니다. 또한, 이 지역은 생물의학 연구개발을 위해 정부 및 민간으로부터 많은 자금 지원을 받고 있어 혁신적인 IHC 기술 및 자동화 플랫폼의 도입이 가속화되고 있습니다. 유리한 상환 정책과 첨단 진단 옵션에 대한 임상의와 환자들의 높은 인식도 시장 확대에 기여하고 있습니다. 엄격한 규제 환경과 IHC 제품의 품질과 신뢰성을 보장하는 견고한 프레임워크는 임상 진단과 급성장하는 제약 및 생명공학 연구 분야에서 신뢰와 보급을 촉진하고 세계 IHC 시장에서 북미의 우위를 확고히 하고 있습니다.
세계의 면역조직화학 시장에 대해 조사했으며, 제품별, 용도별, 최종사용자별, 지역별 동향, 시장 진입 기업 프로파일 등의 정보를 정리하여 전해드립니다.
목차
제1장 소개
제2장 조사 방법
제3장 주요 요약
제4장 주요 인사이트
제5장 시장 개요
- 소개
- 시장 역학
- 고객의 비즈니스에 영향을 미치는 동향/혼란
- 가격 분석
- 기술 분석
- 특허 분석
- 무역 분석
- 밸류체인 분석
- 생태계 분석
- Porter's Five Forces 분석
- 주요 이해관계자와 구입 기준
- 관세 및 규제 분석
- 2025-2026년의 주요 회의와 이벤트
- 투자와 자금 조달 시나리오
- AI/생성형 AI가 면역조직화학 시장에 미치는 영향
- 2025년 미국 관세가 면역조직화학 시장에 미치는 영향
제6장 면역조직화학 시장(제공별)
- 소개
- 항체
- 시약
- 키트
- 기기와 소프트웨어
- 서비스
제7장 면역조직화학 시장(용도별)
- 소개
- 진단
- 연구
- 법의학
제8장 면역조직화학제품 시장(최종사용자별)
- 소개
- 병원 및 진단 검사실
- 학술기관 및 CRO
- 기타
제9장 면역조직화학 서비스 시장(최종사용자별)
- 소개
- 제약회사 및 바이오의약품 회사
- 학술기관
제10장 면역조직화학 시장(지역별)
- 소개
- 북미
- 북미의 거시경제 전망
- 미국
- 캐나다
- 유럽
- 유럽의 거시경제 전망
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타
- 아시아태평양
- 아시아태평양의 거시경제 전망
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타
- 라틴아메리카
- 라틴아메리카의 거시경제 전망
- 브라질
- 멕시코
- 기타
- 중동
- 중동의 거시경제 전망
- GCC 국가
- 기타
- 아프리카
- 암 환자 급증과 헬스케어에 대한 거액의 투자가 시장을 견인
- 아프리카의 거시경제 전망
제11장 경쟁 구도
- 소개
- 주요 진출 기업의 전략/강점
- 매출 분석, 2020-2024년
- 시장 점유율 분석, 2024년
- 기업 평가 매트릭스 : 주요 진출 기업, 2024년
- 기업 평가 매트릭스 : 스타트업/중소기업, 2024년
- 기업 평가와 재무 지표
- 브랜드/제품 비교
- 경쟁 시나리오
제12장 기업 개요
- 주요 진출 기업
- F. HOFFMANN-LA ROCHE LTD.
- DANAHER CORPORATION
- AGILENT TECHNOLOGIES, INC.
- PHC HOLDINGS CORPORATION
- THERMO FISHER SCIENTIFIC INC.
- MERCK KGAA
- BIO-RAD LABORATORIES, INC.
- BIO-TECHNE
- BECTON, DICKINSON AND COMPANY(BD)
- TAKARA BIO INC.
- ENZO BIOCHEM INC.
- SINO BIOLOGICAL, INC.
- SAKURA FINETEK JAPAN CO., LTD.
- CELL SIGNALING TECHNOLOGY, INC.
- BIO SB
- MILTENYI BIOTEC
- ORIGENE TECHNOLOGIES, INC.
- 기타 기업
- EAGLEBIO
- BIOCARE MEDICAL, LLC
- ELABSCIENCE
- BIOGENEX
- DIAGNOSTIC BIOSYSTEMS INC.
- HISTO-LINE LABORATORIES
- ROCKLAND IMMUNOCHEMICALS, INC.
- CANDOR BIOSCIENCE GMBH
- GENEMED BIOTECHNOLOGIES, INC.
제13장 부록
KSM 25.09.29The immunohistochemistry market is expected to reach USD 5.14 billion in 2030 from USD 3.55 billion in 2024, at a CAGR of 7.6% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Offering, Application, End User |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa |
Factors propelling the growth of the market are the increased prevalence of cancer cases among the growing geriatric population and the rising application of IHC tests for the diagnosis and management of cancer. Moreover, the development of companion diagnostics (CDx) and its integration with IHC technology to develop personalized medicine further support the expansion of the market.
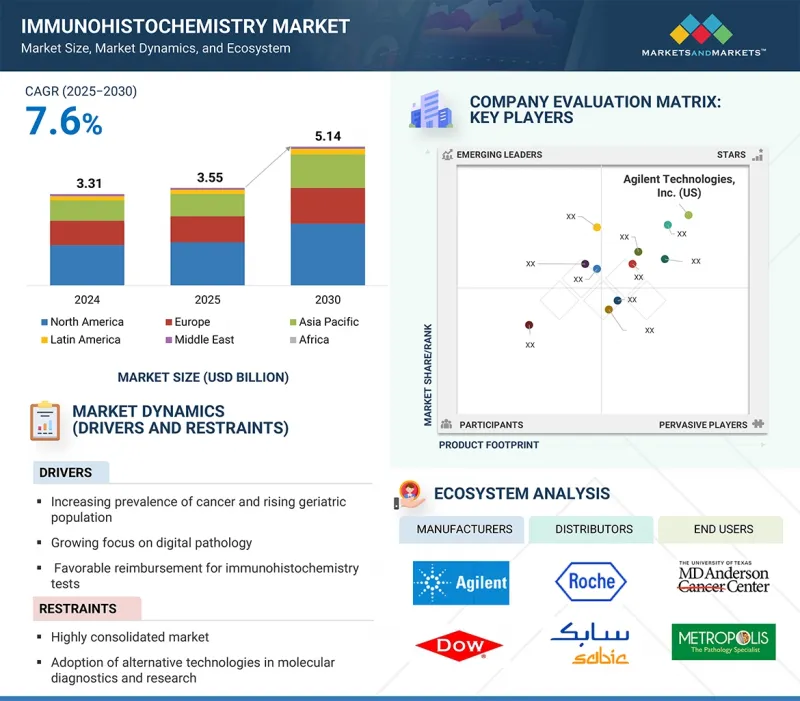
The histological stain segment accounted for the largest share of the immunohistochemistry reagents segment in 2024.
The market is segmented into histological stains, blocking sera and reagents, chromogenic substrates, fixation reagents, diluents, organic solvents, proteolytic enzymes, and other reagents. In 2024, the histological stains segment accounted for the largest share of the immunohistochemistry market. This is due to their primary application being in immunohistochemistry, a powerful technique where stains are used to visualize and differentiate specific cellular components, proteins, and structures within tissue samples by leveraging antigen-antibody interactions. This allows for the precise localization of biomarkers critical for diagnosis and research. Furthermore, the utility of histological stains is expanding through advanced techniques like multiplex staining, also known as multiple staining. This method employs several stains simultaneously to detect multiple targets within a single tissue section. This is particularly valuable in fields like immuno-oncology as it conserves precious patient samples while providing a comprehensive view of the complex tumor microenvironment. A major driver for this market remains its pivotal function in cancer diagnosis, where pathologists use these stains to distinguish between normal and abnormal tissue morphology, thereby determining tumor grade and type.
In 2024, by application, the research applications segment accounted for the second-largest share of the market.
The immunohistochemistry market is segmented into diagnostic applications, research applications, and forensic applications. In 2024, the research applications segment accounted for the second-largest share of the immunohistochemistry market. IHC technology is a cornerstone of modern biomedical investigation, particularly in anti-tumor drug development and biomarker discovery. It provides researchers with an unparalleled ability to visualize the expression and precise subcellular location of specific proteins within the contextual architecture of tissue. This is fundamental to understanding the complex cellular mechanisms that underpin cancer and other diseases. For instance, in the exploration of the cancer proteome, IHC is instrumental for validating new drug targets identified through genomic or proteomic screening, confirming their presence and relevance in actual tumor tissues. The role of IHC in research extends across numerous scientific disciplines. In developmental biology and embryological studies, it allows for mapping key regulatory proteins during different stages of development, offering insights crucial for understanding morphogenesis and congenital disorders. Similarly, in stem cell research, IHC is used to monitor markers of proliferation and differentiation, providing essential data on stem cell behavior and therapeutic potential. The continuous investment in R&D by pharmaceutical and biotechnology companies, coupled with the global push towards personalized medicine, ensures that the research application segment will remain a dynamic and expanding component of the immunohistochemistry market.
.
In 2024, North America accounted for the largest share of the immunohistochemistry market.
North America accounted for the largest share of the immunohistochemistry market in 2024. This leadership position is underpinned by various factors, including the region's highly advanced healthcare infrastructure, substantial healthcare expenditure, and the robust presence of key market players and leading research institutions. The US, in particular, serves as the primary driver of this growth, fueled by a high prevalence of chronic diseases, most notably cancer. The significant and rising incidence of various cancers necessitates sophisticated and accurate diagnostic tools, with IHC being a cornerstone of modern pathology for tumor classification and biomarker analysis. Furthermore, the region benefits from extensive government and private funding for biomedical research and development, which accelerates the adoption of innovative IHC technologies and automated platforms. Favorable reimbursement policies and a high level of awareness among clinicians and patients regarding advanced diagnostic options also contribute to the market's expansion. The strong regulatory framework, while stringent, ensures the quality and reliability of IHC products, fostering trust and widespread use in both clinical diagnostics and the burgeoning pharmaceutical and biotechnology research sectors, solidifying the preeminent standing of North America in the global IHC landscape.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Tier 1-25%, Tier 2-35%, and Tier 3- 40%
- By Designation: C-level Executives - 55%, Directors- 20%, and Others- 25%
- By Region: North America -45%, Europe - 20%, Asia Pacific -20%, Latin America -10%, the Middle East- 3%, and Africa-2%
F. Hoffmann-La Roche Ltd (Switzerland), Danaher Corporation (US), Agilent Technologies, Inc. (US), Thermo Fisher Scientific Inc. (US), Merck KGaA (Germany), Bio-Rad Laboratories, Inc. (US), Bio-Techne (US), Becton, Dickinson and Company (US), Takara Bio Inc. (Japan), PHC Holdings Corporation (Japan), Enzo Biochem Inc. (US), Sino Biological, Inc. (China), OriGene Technologies, Inc. (US), Cell Signaling Technology, Inc. (US), Bio SB (US), Miltenyi Biotec (Germany), Sakura Finetek (US), EagleBio (US), Biocare Medical LLC (US), Elabscience BioInnovation Inc. (US), Bio-Genex (US), Diagnostic BioSystems Inc. (US), Histo-Line Laboratories (Italy), Rockland Immunochemicals, Inc. (US), and CANDOR Bioscience GmbH (Germany) are some of the key companies offering immunohistochemistry products.
Research Coverage
This research report categorizes the immunohistochemistry market by Product & Service, Product (Antibodies [Primary antibodies, secondary antibodies], [Clonality {Monoclonal, Polyclonal}], [Regulatory Class {Class I/II Assay Antibodies, Class III Assay Antibodies}], Reagents [blocking sera and reagents, chromogenic substrates, fixation reagents, diluents, organic solvents, proteolytic enzymes, other reagents], Kits [Human Immunohistochemistry Kits, Animal Immunohistochemistry Kits], Instruments & Software (IHC Stainers), [Sample Preparation Instruments {Tissue Processors, Microtomes, Other Sample Preparation Instruments}], (Image Analysis Instruments), Service, Application (Diagnostic Applications [Cancer, Infectious Diseases, Nephrological Diseases, Autoimmune Diseases, Neurological Diseases, Other Diseases] Research Applications [Drug Development and testing, Other Research Applications], Forensic Applications}, End User for Product (Hospitals & Diagnostic Laboratories, Academic & Research Institutes and Contract Research Organizations, Other End Users), End User for Service (Pharmaceutical & Biotechnology Companies and Academic Institutions) and by region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa). The scope of the report covers detailed information regarding the major factors, such as drivers, challenges, opportunities, and restraints influencing the growth of the immunohistochemistry market. A detailed analysis of the key industry players has been done to provide insights into their business overview, service portfolio, key strategies such as collaborations, partnerships, expansions, agreements, acquisitions, and recent developments associated with the immunohistochemistry market. This report covers a competitive analysis of top players and upcoming startups in the immunohistochemistry market ecosystem.
The scope of the report covers detailed information regarding the primary factors, such as drivers, restraints, challenges, and opportunities, influencing the growth of the immunohistochemistry market. A thorough analysis of the key industry players has been conducted to provide insights into their business overview, solutions, and services; key strategies; new product & service launches, acquisitions, and recent developments associated with the immunohistochemistry market. This report covers the competitive analysis of upcoming startups in the immunohistochemistry market ecosystem.
Key Benefits of Buying the Report
This report provides a detailed picture of the immunohistochemistry market. It aims at estimating the size and future growth potential of the market across different segments such as the product, application, end user, and region. The report also includes an in-depth competitive analysis of the key market players along with their company profiles recent developments and key market strategies.
The report provides insights on the following pointers:
Analysis of key drivers (Rising Geriatric Population and Increasing Cancer Prevalence, Innovations and advancements in Immunohistochemistry (IHC) Technology, Reimbursement Coverage for Immunohistochemistry (IHC) Tests, Rising Adoption of Digital Pathology), restraint (Adoption of alternative technologies and a high degree of consolidation), opportunities (Rising demand for personalized medicine, Growing adoption of companion diagnostics, Growth opportunities in emerging countries, Integration of artificial intelligence in IHC), Challenges (Stringent regulatory requirements, Lack of Standardization)
- Product Development/Innovation: Detailed insights on newly launched product, and technological assessment of the Immunohistochemistry market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the Immunohistochemistry market across varied regions.
- Market Diversification: Exhaustive information about new, untapped geographies, recent developments, and investments in the Immunohistochemistry market
Competitive Assessment: In-depth assessment of market shares, growth strategies and product offerings of leading players like F. Hoffman-La Roche Ltd (Switzerland), Danaher Corporation (US), and Agilent Technologies, Inc. (US) among others in the Immunohistochemistry market. The report also helps stakeholders understand the pulse of the IHC market and provides them with information on key market drivers, restraints, challenges, and opportunities. A detailed analysis of the key industry players has been done to provide insights into their key strategies, product launches/ approvals, pipeline analysis, acquisitions, partnerships, agreements, collaborations, other recent developments, investment and funding activities, brand/product comparative analysis, and vendor valuation and financial metrics of the GLP-1 analogues market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
- 1.5 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key sources of secondary data
- 2.1.1.2 Key objectives of secondary research
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Breakdown of primaries
- 2.1.2.2 Key objectives of primary research
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 GLOBAL MARKET SIZE ESTIMATION
- 2.2.1.1 Revenue share analysis (bottom-up approach)
- 2.2.1.2 Secondary data
- 2.2.1.3 Primary interviews and MnM repository analysis
- 2.2.2 INSIGHTS FROM PRIMARY EXPERTS
- 2.2.3 SEGMENTAL MARKET ASSESSMENT (TOP-DOWN APPROACH)
- 2.2.1 GLOBAL MARKET SIZE ESTIMATION
- 2.3 MARKET GROWTH RATE PROJECTION
- 2.4 DATA TRIANGULATION
- 2.5 STUDY ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
- 3.1 KEY INSIGHTS & MARKET HIGHLIGHTS
- 3.2 STRATEGIC IMPERATIVES FOR STAKEHOLDERS
- 3.3 DISRUPTIVE TRENDS SHAPING IMMUNOHISTOCHEMISTRY MARKET
- 3.4 HIGH-GROWTH SEGMENTS & EMERGING FRONTIERS
- 3.5 GLOBAL MARKET SIZE, GROWTH RATE, AND FORECAST
4 PREMIUM INSIGHTS
- 4.1 IMMUNOHISTOCHEMISTRY MARKET OVERVIEW
- 4.2 NORTH AMERICA: IMMUNOHISTOCHEMISTRY MARKET, BY APPLICATION AND COUNTRY, 2024
- 4.3 IMMUNOHISTOCHEMISTRY PRODUCTS MARKET SHARE, BY END USER, 2024
- 4.4 IMMUNOHISTOCHEMISTRY MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.5 UNMET NEEDS & WHITE SPACES
- 4.6 INTERCONNECTED MARKETS & CROSS-SECTOR OPPORTUNITIES
- 4.7 EMERGING BUSINESS MODELS & ECOSYSTEM SHIFTS
- 4.8 STRATEGIC MOVES BY TIER-1/2/3 PLAYERS
- 4.9 VC/PRIVATE EQUITY INVESTMENT TRENDS
- 4.10 SUSTAINABILITY IMPACT & REGULATORY POLICY INITIATIVES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Increasing adoption of companion diagnostics
- 5.2.1.2 Favorable reimbursement coverage for immunohistochemistry tests
- 5.2.1.3 Growing focus on digital pathology
- 5.2.2 RESTRAINTS
- 5.2.2.1 Adoption of alternative technologies in molecular diagnostics and research
- 5.2.2.2 Highly consolidated global market
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Rising demand for precision/personalized medicines
- 5.2.3.2 High growth opportunities in emerging economies
- 5.2.3.3 Integration of AI in immunohistochemistry
- 5.2.4 CHALLENGES
- 5.2.4.1 Stringent regulatory requirements for tissue diagnostic instruments and consumables
- 5.2.4.2 Lack of preanalytical and analytical method standardization
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- 5.4 PRICING ANALYSIS
- 5.4.1 AVERAGE SELLING PRICE TREND OF IMMUNOHISTOCHEMISTRY PRODUCTS, BY KEY PLAYER, 2022-2024
- 5.4.2 AVERAGE SELLING PRICE TREND OF IMMUNOHISTOCHEMISTRY PRODUCTS, BY REGION, 2022-2024
- 5.5 TECHNOLOGY ANALYSIS
- 5.5.1 KEY TECHNOLOGIES
- 5.5.1.1 Automated immunohistochemistry systems
- 5.5.1.2 Multiplex immunohistochemistry
- 5.5.2 COMPLEMENTARY TECHNOLOGIES
- 5.5.2.1 Chromogenic detection
- 5.5.2.2 Fluorescent detection
- 5.5.3 ADJACENT TECHNOLOGIES
- 5.5.3.1 In situ hybridization
- 5.5.1 KEY TECHNOLOGIES
- 5.6 PATENT ANALYSIS
- 5.6.1 METHODOLOGY
- 5.6.2 NUMBER OF PATENTS FILED, BY DOCUMENT TYPE
- 5.6.3 LIST OF KEY PATENTS
- 5.7 TRADE ANALYSIS
- 5.7.1 IMPORT DATA FOR HS CODE 3822.00, 2020-2024
- 5.7.2 EXPORT DATA FOR HS CODE 3822.00, 2020-2024
- 5.8 VALUE CHAIN ANALYSIS
- 5.9 ECOSYSTEM ANALYSIS
- 5.9.1 ROLE IN ECOSYSTEM
- 5.10 PORTER'S FIVE FORCES ANALYSIS
- 5.10.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.10.2 BARGAINING POWER OF SUPPLIERS
- 5.10.3 BARGAINING POWER OF BUYERS
- 5.10.4 THREAT OF SUBSTITUTES
- 5.10.5 THREAT OF NEW ENTRANTS
- 5.11 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.11.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.11.2 KEY BUYING CRITERIA
- 5.12 TARIFF & REGULATORY ANALYSIS
- 5.12.1 TARIFF DATA ANALYSIS
- 5.12.2 REGULATORY LANDSCAPE
- 5.12.2.1 North America
- 5.12.2.1.1 US
- 5.12.2.1.2 Canada
- 5.12.2.2 Europe
- 5.12.2.3 Asia Pacific
- 5.12.2.3.1 Japan
- 5.12.2.3.2 China
- 5.12.2.3.3 India
- 5.12.2.4 Latin America
- 5.12.2.4.1 Brazil
- 5.12.2.1 North America
- 5.12.3 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.13 KEY CONFERENCES & EVENTS, 2025-2026
- 5.14 INVESTMENT & FUNDING SCENARIO
- 5.15 IMPACT OF AI/GEN AI ON IMMUNOHISTOCHEMISTRY MARKET
- 5.16 IMPACT OF 2025 US TARIFF ON IMMUNOHISTOCHEMISTRY MARKET
- 5.16.1 KEY TARIFF RATES
- 5.16.2 PRICE IMPACT ANALYSIS
- 5.16.3 IMPACT ON KEY COUNTRIES/REGIONS
- 5.16.3.1 North America
- 5.16.3.1.1 US
- 5.16.3.2 Europe
- 5.16.3.3 Asia Pacific
- 5.16.3.4 Rest of the World
- 5.16.3.1 North America
- 5.16.4 IMPACT ON END-USE INDUSTRIES
- 5.16.4.1 Pharmaceutical & biopharmaceutical companies
- 5.16.4.2 Hospitals & clinics
- 5.16.4.3 Academic & research institutes
- 5.16.4.4 Contract research organizations
6 IMMUNOHISTOCHEMISTRY MARKET, BY OFFERING
- 6.1 INTRODUCTION
- 6.2 ANTIBODIES
- 6.2.1 IMMUNOHISTOCHEMISTRY ANTIBODIES MARKET, BY TYPE
- 6.2.1.1 Primary antibodies
- 6.2.1.1.1 Increasing adoption of targeted immunotherapy and custom antibody development to drive segment
- 6.2.1.2 Secondary antibodies
- 6.2.1.2.1 Better signal amplification and easier manufacturing process to fuel segment growth
- 6.2.1.1 Primary antibodies
- 6.2.2 IMMUNOHISTOCHEMISTRY ANTIBODIES MARKET, BY CLONALITY
- 6.2.2.1 Monoclonal antibodies
- 6.2.2.1.1 Increased applications in cancer diagnostics to support segment growth
- 6.2.2.2 Polyclonal antibodies
- 6.2.2.2.1 Better sensitivity and higher resistance to changes in antigen conformation to augment segment growth
- 6.2.2.1 Monoclonal antibodies
- 6.2.3 IMMUNOHISTOCHEMISTRY ANTIBODIES MARKET, BY REGULATORY CLASS
- 6.2.3.1 Class I/II assay antibodies
- 6.2.3.1.1 Focus on precision medicine and standardized reagents to favor segment growth
- 6.2.3.2 Class III assay antibodies
- 6.2.3.2.1 Expansion of targeted therapies to augment segment growth
- 6.2.3.1 Class I/II assay antibodies
- 6.2.1 IMMUNOHISTOCHEMISTRY ANTIBODIES MARKET, BY TYPE
- 6.3 REAGENTS
- 6.3.1 BLOCKING SERA & REAGENTS
- 6.3.1.1 Need to prevent non-specific binding and provide accurate outcomes to drive adoption
- 6.3.2 CHROMOGENIC SUBSTRATES
- 6.3.2.1 Rapid detection and faster diagnosis to propel segment growth
- 6.3.3 FIXATION REAGENTS
- 6.3.3.1 Increased focus on retaining tissue morphology and antigenicity of target molecules to support adoption
- 6.3.4 ORGANIC SOLVENTS
- 6.3.4.1 Need to prevent physical damage in specimens and improve clarity in tissue sample visualization to drive market
- 6.3.5 PROTEOLYTIC ENZYMES
- 6.3.5.1 Proteolytic enzymes to improve accessibility of target antibodies and DNA
- 6.3.6 DILUENTS
- 6.3.6.1 Need for reducing non-specific background staining during immunostaining to aid segment growth
- 6.3.7 OTHER REAGENTS
- 6.3.1 BLOCKING SERA & REAGENTS
- 6.4 KITS
- 6.4.1 HUMAN IMMUNOHISTOCHEMISTRY KITS
- 6.4.1.1 Increasing focus on cancer research to fuel segment growth
- 6.4.2 ANIMAL IMMUNOHISTOCHEMISTRY KITS
- 6.4.2.1 Rising focus on drug safety and efficacy in preclinical drug testing to boost segment growth
- 6.4.1 HUMAN IMMUNOHISTOCHEMISTRY KITS
- 6.5 INSTRUMENTS & SOFTWARE
- 6.5.1 IHC STAINERS
- 6.5.1.1 Increased availability of automated IHC stainers to fuel segment growth
- 6.5.2 SAMPLE PREPARATION INSTRUMENTS
- 6.5.2.1 Tissue processors
- 6.5.2.1.1 Rising biopsy volumes and increasing workflow automation to fuel segment growth
- 6.5.2.2 Microtomes
- 6.5.2.2.1 High precision demand and increased workforce challenges to aid segment growth
- 6.5.2.3 Other sample preparation instruments
- 6.5.2.1 Tissue processors
- 6.5.3 IMAGE ANALYSIS INSTRUMENTS
- 6.5.3.1 Escalating demand for objective and reproducible quantification of IHC-stained tissue to boost segment growth
- 6.5.1 IHC STAINERS
- 6.6 SERVICES
- 6.6.1 INCREASED COMPLEXITY OF BIOMARKER REQUIREMENTS AND EXPANSION OF GLOBAL CLINICAL TRIALS TO AID MARKET GROWTH
7 IMMUNOHISTOCHEMISTRY MARKET, BY APPLICATION
- 7.1 INTRODUCTION
- 7.2 DIAGNOSTIC APPLICATIONS
- 7.2.1 CANCER
- 7.2.1.1 Automation, AI-enhanced quantitation, and multiplexing to accelerate global cancer IHC adoption
- 7.2.2 INFECTIOUS DISEASES
- 7.2.2.1 Multiplex pathogen panels, automation, and AI-driven imaging to boost market growth
- 7.2.3 AUTOIMMUNE DISEASES
- 7.2.3.1 Focus on AI-powered multiplex imaging and automated analysis to propel market growth
- 7.2.4 NEPHROLOGICAL DISEASES
- 7.2.4.1 Better understanding of novel renal biomarkers and virtual multiplexing to accelerate market growth
- 7.2.5 NEUROLOGICAL DISEASES
- 7.2.5.1 Advances in neuroimmunopathology, novel biomarker discovery, multiplexing, and AI-supported image analysis to drive market
- 7.2.6 OTHER DIAGNOSTIC APPLICATIONS
- 7.2.1 CANCER
- 7.3 RESEARCH APPLICATIONS
- 7.3.1 DRUG DEVELOPMENT & TESTING
- 7.3.1.1 AI-enabled quantitation, regulatory support, and organ-on-chip integration to propel growth in drug development
- 7.3.2 OTHER RESEARCH APPLICATIONS
- 7.3.1 DRUG DEVELOPMENT & TESTING
- 7.4 FORENSIC APPLICATIONS
- 7.4.1 ADVANCEMENTS IN VALIDATED INJURY AND DEATH MARKERS WITH IHC INTEGRATION IN FORENSIC IMAGING TO DRIVE MARKET
8 IMMUNOHISTOCHEMISTRY PRODUCTS MARKET, BY END USER
- 8.1 INTRODUCTION
- 8.2 HOSPITALS & DIAGNOSTIC LABORATORIES
- 8.2.1 EXPANSION OF DIAGNOSTIC INFRASTRUCTURE AND DIGITAL AUTOMATION TO DRIVE GLOBAL MARKET GROWTH
- 8.3 ACADEMIC INSTITUTIONS AND CONTRACT RESEARCH ORGANIZATIONS
- 8.3.1 INNOVATION IN MULTIPLEX AND AI-ENABLED IMMUNOHISTOCHEMISTRY TO FUEL GLOBAL MARKET EXPANSION
- 8.4 OTHER END USERS
9 IMMUNOHISTOCHEMISTRY SERVICES MARKET, BY END USER
- 9.1 INTRODUCTION
- 9.2 PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES
- 9.2.1 INTEGRATION OF MULTIPLEX IHC SERVICES WITH REGULATORY-CLEARED WORKFLOWS TO AUGMENT MARKET GROWTH
- 9.3 ACADEMIC INSTITUTIONS
- 9.3.1 ADOPTION OF MULTIPLEX SPATIAL IMMUNOHISTOCHEMISTRY AND DIGITAL PATHOLOGY TO BOOST MARKET GROWTH
10 IMMUNOHISTOCHEMISTRY MARKET, BY REGION
- 10.1 INTRODUCTION
- 10.2 NORTH AMERICA
- 10.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 10.2.2 US
- 10.2.2.1 US to dominate North American immunohistochemistry market during forecast period
- 10.2.3 CANADA
- 10.2.3.1 High incidence of cancer and increased demand for advanced diagnostics to propel market growth
- 10.3 EUROPE
- 10.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 10.3.2 GERMANY
- 10.3.2.1 High investments in life science R&D and favorable reimbursement policies to drive market
- 10.3.3 UK
- 10.3.3.1 Rising prevalence of cancer and increasing government funding in healthcare research to augment market growth
- 10.3.4 FRANCE
- 10.3.4.1 Increased research in cancer diagnosis and treatment to support market growth
- 10.3.5 ITALY
- 10.3.5.1 Advanced healthcare infrastructure and high geriatric population to fuel market growth
- 10.3.6 SPAIN
- 10.3.6.1 Increased demand for personalized medicines to boost adoption of immunohistochemistry products
- 10.3.7 REST OF EUROPE
- 10.4 ASIA PACIFIC
- 10.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 10.4.2 CHINA
- 10.4.2.1 Rising healthcare investments and increasing cancer burden to spur market growth
- 10.4.3 JAPAN
- 10.4.3.1 High geriatric population and increased government healthcare expenditure to support market growth
- 10.4.4 INDIA
- 10.4.4.1 Need for high-end pathology and diagnostic services to propel market growth
- 10.4.5 AUSTRALIA
- 10.4.5.1 Increased patient population and high demand for quality healthcare to aid market growth
- 10.4.6 SOUTH KOREA
- 10.4.6.1 Focus on precision healthcare and personalized medicines to drive market
- 10.4.7 REST OF ASIA PACIFIC
- 10.5 LATIN AMERICA
- 10.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 10.5.2 BRAZIL
- 10.5.2.1 Increasing incidence of chronic lifestyle diseases and rising investments by private companies to fuel market growth
- 10.5.3 MEXICO
- 10.5.3.1 Increasing research initiatives and rising cancer cases to drive market
- 10.5.4 REST OF LATIN AMERICA
- 10.6 MIDDLE EAST
- 10.6.1 MACROECONOMIC OUTLOOK FOR MIDDLE EAST
- 10.6.2 GCC COUNTRIES
- 10.6.2.1 Kingdom of Saudi Arabia
- 10.6.2.1.1 Growing cancer burden and increasing focus on oncology-based research programs to augment market growth
- 10.6.2.2 UAE
- 10.6.2.2.1 Favorable government initiatives and high investments in healthcare infrastructure to fuel market growth
- 10.6.2.3 Rest of GCC Countries
- 10.6.2.1 Kingdom of Saudi Arabia
- 10.6.3 REST OF MIDDLE EAST
- 10.7 AFRICA
- 10.7.1 RAPID RISE IN CANCER CASES AND HIGH HEALTHCARE INVESTMENTS TO DRIVE MARKET
- 10.7.2 MACROECONOMIC OUTLOOK FOR AFRICA
11 COMPETITIVE LANDSCAPE
- 11.1 INTRODUCTION
- 11.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN IMMUNOHISTOCHEMISTRY MARKET
- 11.3 REVENUE ANALYSIS, 2020-2024
- 11.4 MARKET SHARE ANALYSIS, 2024
- 11.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 11.5.1 STARS
- 11.5.2 EMERGING LEADERS
- 11.5.3 PERVASIVE PLAYERS
- 11.5.4 PARTICIPANTS
- 11.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 11.5.5.1 Company footprint
- 11.5.5.2 Region footprint
- 11.5.5.3 Offering footprint
- 11.5.5.4 Application footprint
- 11.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 11.6.1 PROGRESSIVE COMPANIES
- 11.6.2 RESPONSIVE COMPANIES
- 11.6.3 DYNAMIC COMPANIES
- 11.6.4 STARTING BLOCKS
- 11.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 11.6.5.1 Detailed list of key startups/SMEs
- 11.6.5.2 Competitive benchmarking of key startups/SMEs
- 11.7 COMPANY VALUATION & FINANCIAL METRICS
- 11.7.1 FINANCIAL METRICS
- 11.7.2 COMPANY VALUATION
- 11.8 BRAND/PRODUCT COMPARISON
- 11.9 COMPETITIVE SCENARIO
- 11.9.1 PRODUCT APPROVALS
- 11.9.2 DEALS
- 11.9.3 EXPANSIONS
- 11.9.4 OTHER DEVELOPMENTS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- 12.1.1 F. HOFFMANN-LA ROCHE LTD.
- 12.1.1.1 Business overview
- 12.1.1.2 Products/Services offered
- 12.1.1.3 Recent developments
- 12.1.1.3.1 Product launches and approvals
- 12.1.1.4 MnM view
- 12.1.1.4.1 Key strengths
- 12.1.1.4.2 Strategic choices
- 12.1.1.4.3 Weaknesses & competitive threats
- 12.1.2 DANAHER CORPORATION
- 12.1.2.1 Business overview
- 12.1.2.2 Products/Services offered
- 12.1.2.3 Recent developments
- 12.1.2.3.1 Product launches and approvals
- 12.1.2.3.2 Deals
- 12.1.2.4 MnM view
- 12.1.2.4.1 Key strengths
- 12.1.2.4.2 Strategic choices
- 12.1.2.4.3 Weaknesses & competitive threats
- 12.1.3 AGILENT TECHNOLOGIES, INC.
- 12.1.3.1 Business overview
- 12.1.3.2 Products/Services offered
- 12.1.3.3 Recent developments
- 12.1.3.3.1 Product approvals
- 12.1.3.3.2 Deals
- 12.1.3.3.3 Expansions
- 12.1.3.4 MnM view
- 12.1.3.4.1 Key strengths
- 12.1.3.4.2 Strategic choices
- 12.1.3.4.3 Weaknesses & competitive threats
- 12.1.4 PHC HOLDINGS CORPORATION
- 12.1.4.1 Business overview
- 12.1.4.2 Products/Services offered
- 12.1.4.3 Recent developments
- 12.1.4.3.1 Deals
- 12.1.4.4 MnM view
- 12.1.4.4.1 Key strengths
- 12.1.4.4.2 Strategic choices
- 12.1.4.4.3 Weaknesses & competitive threats
- 12.1.5 THERMO FISHER SCIENTIFIC INC.
- 12.1.5.1 Business overview
- 12.1.5.2 Products/Services offered
- 12.1.5.3 MnM view
- 12.1.5.3.1 Key strengths
- 12.1.5.3.2 Strategic choices
- 12.1.5.3.3 Weaknesses & competitive threats
- 12.1.6 MERCK KGAA
- 12.1.6.1 Business overview
- 12.1.6.2 Products/Services offered
- 12.1.7 BIO-RAD LABORATORIES, INC.
- 12.1.7.1 Business overview
- 12.1.7.2 Products/Services offered
- 12.1.8 BIO-TECHNE
- 12.1.8.1 Business overview
- 12.1.8.2 Products/Services offered
- 12.1.8.3 Recent developments
- 12.1.8.3.1 Deals
- 12.1.8.3.2 Other developments
- 12.1.9 BECTON, DICKINSON AND COMPANY (BD)
- 12.1.9.1 Business overview
- 12.1.9.2 Products/Services offered
- 12.1.10 TAKARA BIO INC.
- 12.1.10.1 Business overview
- 12.1.10.2 Products/Services offered
- 12.1.11 ENZO BIOCHEM INC.
- 12.1.11.1 Business overview
- 12.1.11.2 Products/Services offered
- 12.1.11.3 Recent developments
- 12.1.11.3.1 Expansions
- 12.1.12 SINO BIOLOGICAL, INC.
- 12.1.12.1 Business overview
- 12.1.12.2 Products/Services offered
- 12.1.13 SAKURA FINETEK JAPAN CO., LTD.
- 12.1.13.1 Business overview
- 12.1.13.2 Products/Services offered
- 12.1.13.3 Recent developments
- 12.1.13.3.1 Product launches
- 12.1.13.3.2 Deals
- 12.1.14 CELL SIGNALING TECHNOLOGY, INC.
- 12.1.14.1 Business overview
- 12.1.14.2 Products/Services offered
- 12.1.14.3 Recent developments
- 12.1.14.3.1 Deals
- 12.1.15 BIO SB
- 12.1.15.1 Business overview
- 12.1.15.2 Products/Services offered
- 12.1.15.3 Recent developments
- 12.1.15.3.1 Product launches
- 12.1.16 MILTENYI BIOTEC
- 12.1.16.1 Business overview
- 12.1.16.2 Products/Services offered
- 12.1.17 ORIGENE TECHNOLOGIES, INC.
- 12.1.17.1 Business overview
- 12.1.17.2 Products/Services offered
- 12.1.1 F. HOFFMANN-LA ROCHE LTD.
- 12.2 OTHER PLAYERS
- 12.2.1 EAGLEBIO
- 12.2.2 BIOCARE MEDICAL, LLC
- 12.2.3 ELABSCIENCE
- 12.2.4 BIOGENEX
- 12.2.5 DIAGNOSTIC BIOSYSTEMS INC.
- 12.2.6 HISTO-LINE LABORATORIES
- 12.2.7 ROCKLAND IMMUNOCHEMICALS, INC.
- 12.2.8 CANDOR BIOSCIENCE GMBH
- 12.2.9 GENEMED BIOTECHNOLOGIES, INC.
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 CUSTOMIZATION OPTIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS



















