
|
시장보고서
상품코드
1906297
의료용 멤브레인 시장(-2030년) : 재료, 프로세스 기술, 용도, 지역별Medical Membranes Market by Material, Process Technology, Application, and Region - Global Forecast to 2030 |
||||||
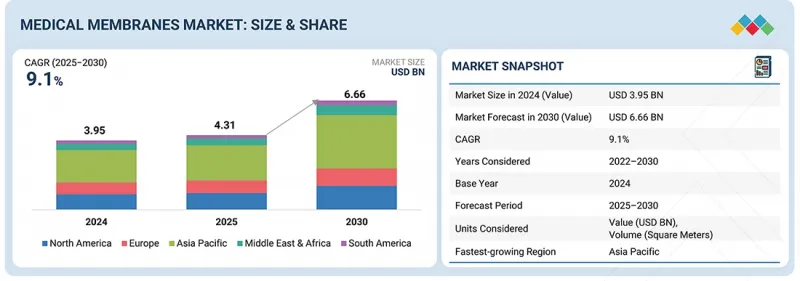
의료용 멤브레인 시장 규모는 예측 기간 중에 CAGR 9.1%로 성장하여 2025년 43억 1,000만 달러에서 2030년에는 66억 6,000만 달러에 이를 전망입니다.
세계 의료용 멤브레인 시장의 성장은 만성 신장 질환 및 당뇨병 환자 증가에 의해 주도되고 있습니다. 생체적합성 향상, 방오 코팅, 나노 여과 능력과 같은 멤브레인 기술의 지속적인 혁신으로 약품 정제, 바이오프로세스, 인공장기의 적용이 가능해졌습니다.
| 조사 범위 | |
|---|---|
| 조사 대상 기간 | 2022-2030년 |
| 기준 연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 단위 | 금액(달러), 체적(평방미터) |
| 부문 | 재료, 프로세스 기술, 용도, 지역 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 남미, 중동 및 아프리카 |
또한, 수술 건수 증가, 첨단 상처 드레싱 재료에 대한 수요, 조직 공학용 발판의 보급도 시장 성장을 뒷받침하고 있습니다. 또한, 바이오 기술 및 제약 제조 분야에서는 교차 오염 방지를 위해 일회용 멤브레인 시스템에 대한 투자가 활발히 이루어지고 있습니다. 또한, 신흥 시장, 특히 아시아태평양의 의료 인프라 구축, 성숙 지역의 고령화, 인공장기 및 재생의료에 대한 대규모 연구 개발도 시장 성장에 기여하고 있습니다.

"예측 기간 동안 폴리불화비닐리덴(PVDF)이 가장 빠르게 성장하는 소재 부문으로 부상할 것"이라고 밝혔습니다.
폴리불화비닐리덴(PVDF)은 우수한 특성으로 인해 시장에서 가장 빠르게 성장하고 있는 분야입니다. PVDF는 내화학성, 열 안정성, 물리적 강도, 저단백질 부착성으로 유명하며, 제약 및 바이오 의약품의 액체 정제, 혈액 여과, 약물 정제, 바이오 의약품의 최종 제품 여과 등에 널리 사용되고 있습니다. 친수성 PVDF 멤브레인은 전 세계 만성 질환 증가, 개인 맞춤형 의료에 대한 관심 증가, 고령화 사회의 진전을 배경으로 의료 진단 및 생물학적 제제 생산에 점점 더 많이 활용되고 있습니다. PVDF의 생체적합성은 조직공학, 창상피복재, 바이러스 제거 등 기술 혁신의 핵심 요소로 PTFE, PESU 등 경쟁 소재에 비해 우위를 점하고 있습니다. 동시에 전염병으로 인한 의약품 생산의 급증과 첨단 여과 기술에 대한 투자 확대도 PVDF 수요를 촉진하고 있습니다.
"예측 기간 동안 한외여과가 가장 큰 공정 기술 부문이 될 것으로 예측됩니다."
한외여과(UF)는 혈액 투석에 필수적인 응용 분야로 전체 시장에서 가장 큰 기술 부문을 차지하고 있습니다. 혈액투석은 300만 명 이상의 말기 신장질환 환자에서 신장 기능의 한계를 극복하고, 요독성 독소를 고도로 제거하면서 필수 단백질을 체내에 유지하여 치료를 실현합니다. UF 멤브레인은 일반적으로 폴리설폰, 폴리에테르설폰 또는 개질된 PES로 제조되며, 분자량 컷오프(5-50kDa)가 정밀하게 대응하여 우수한 생체적합성, 높은 플럭스율, 우수한 혈액적합성을 발휘합니다. 따라서 투석 클리닉과 중환자실(ICU)의 정화 표준이 되고 있습니다. 의료용 멤브레인 시장의 급격한 성장은 신장 치료 이외의 분야에서 한외여과(UF)의 사용 확대에 힘입은 바 큽니다. UF는 미생물학 및 제약 다운스트림 공정에서 단클론 항체, 백신, 재조합 단백질 등의 완충액 농축, 여과 및 교환에 널리 적용되고 있으며, 바이오 의약품의 부상과 함께 급속히 증가하고 있습니다. 낮은 막간압력에서의 펌핑 능력과 오염을 줄이는 고도의 표면 개질 기술 채택으로 UF는 대용량, 고부가가치 의료용도에서 한외여과, 역삼투압, 나노여과보다 더 선호되는 기술입니다.
"유럽은 예측 기간 동안 두 번째 규모에 이를 것으로 예측됩니다."
유럽은 의료용 멤브레인 시장에서 두 번째로 큰 규모를 자랑합니다. 이 지역 시장은 우수한 의료 시스템, 1인당 신장 치료에 대한 높은 지출, 만성 신장 질환 유병률이 높은 노인 인구가 많다는 점 등이 시장을 견인하고 있습니다. 또한, 독일, 프랑스, 이탈리아, 스위스에 기반을 둔 주요 멤브레인 제조업체들이 풍부한 R&D 역량과 생산 능력을 보유하고 있는 것도 이 지역 시장을 뒷받침하는 요인으로 작용하고 있습니다. 엄격한 EU 규제 기준에 따라 최고 품질의 제품만 시장에 유통됩니다. 그러나 이는 신규 시장 진출기업에게는 장벽이 될 수 있습니다. 또한, 투석 치료의 광범위한 보험 적용, 급성장하는 바이오로직스 분야의 일회용 바이오프로세싱 시스템 보급, 재생의료 및 생체 인공장기 등의 분야에 대한 공공 및 민간 투자 확대가 제품 수요에 기여하고 있습니다.
세계의 의료용 멤브레인(Medical Membrane) 시장을 조사했으며, 시장 개요, 시장 성장에 영향을 미치는 각종 영향요인 분석, 기술 및 특허 동향, 법 및 규제 환경, 사례 분석, 시장 규모 추이 및 예측, 각종 부문별/지역별/주요 국가별 상세 분석, 경쟁 구도, 주요 기업 개요 등의 정보를 정리하여 전해드립니다.
자주 묻는 질문
목차
제1장 서론
제2장 주요 요약
제3장 프리미엄 인사이트
제4장 시장 개요
- 시장 역학
- 성장 촉진요인
- 성장 억제요인
- 기회
- 과제
- 미충족 요구와 화이트 스페이스
- 관련 시장·타업종과의 분야 횡단적 기회
- 새로운 비즈니스 모델과 에코시스템 변화
- Tier1/2/3 기업의 전략적 움직임
제5장 업계 동향
- Porter의 Five Forces 분석
- 거시경제 지표
- 무역 분석
- 주요 컨퍼런스 및 이벤트
- 고객 사업에 영향을 미치는 동향과 파괴적 변화
- 투자 및 자금조달 시나리오
- 사례 연구 분석
- 2025년 미국 관세
제6장 기술, 특허, 디지털, AI 도입에 의한 전략적 파괴
- 주요 신기술
- 세포막 코팅 나노입자(CNPS)
- 생체 적합성 및 생분해성 복합막
- 보완적 기술
- 표면 개질 및 항균·방오코팅
- 기술/제품 로드맵
- 특허 분석
- 향후 응용
- AI/생성형 AI가 의료용 멤브레인 시장에 미치는 영향
- 성공 사례와 실세계에의 응용
제7장 지속가능성과 규제 상황
- 지역 규제와 컴플라이언스
- 지속가능성 이니셔티브
- 지속가능성에 대한 영향과 규제 정책 대처
- 인증, 라벨, 환경기준
제8장 고객 상황과 구매 행동
- 의사결정 프로세스
- 주요 이해관계자와 구입 기준
- 채택 장벽과 내부 과제
- 다양한 용도에서의 미충족 요구
- 시장 수익성
제9장 의료용 멤브레인 시장 : 재료별
- 폴리설폰(PSU) 및 폴리에테르술폰(PESU)
- 폴리 불화 비닐리덴(PVDF)
- 소수성 PVDF 멤브레인
- 폴리테트라플루오로에틸렌(PTFE)
- 폴리프로필렌(PP)
- 폴리 아크릴로니트릴(PAN)
- 폴리아미드(PA)
- 개질 아크릴
- 기타
제10장 의료용 멤브레인 시장 : 프로세스 기술별
- 한외여과
- 나노여과
- 마이크로 여과
- 기타
제11장 의료용 멤브레인 시장 : 용도별
- 의약품 여과
- 혈액 투석
- 약물 전달
- 정맥주사 혼합 및 무균 여과
- 막형 인공폐
- 혈장분리막
- 기타
제12장 의료용 멤브레인 시장 : 지역별
- 아시아태평양
- 중국
- 인도
- 일본
- 한국
- 기타
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타
- 중동 및 아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
- 남아프리카공화국
- 기타
- 남미
- 브라질
- 아르헨티나
- 기타
제13장 경쟁 구도
- 주요 시장 진출기업의 전략/강점
- 매출 분석
- 시장 점유율 분석
- 기업 평가와 재무 지표
- 제품 비교
- 기업 평가 매트릭스 : 주요 기업
- 기업 평가 매트릭스 : 스타트업/중소기업
- 경쟁 시나리오
제14장 기업 개요
- 주요 기업
- ASAHI KASEI CORPORATION
- MANN+HUMMEL
- SARTORIUS AG
- MERCK KGAA
- SOLVENTUM
- CYTIVA(DANAHER CORPORATION)
- W. L. GORE & ASSOCIATES, INC.
- KOVALUS SEPARATION SOLUTIONS
- COBETTER
- POREX
- 기타 기업
- ADVANCED MICRODEVICES PVT. LTD.
- AMERICAN MEMBRANE CORPORATION
- AMS MEMTECH TECHNOLOGY(ZHEJIANG) CO., LTD
- APPLIED MEMBRANE TECHNOLOGY, INC.
- GRAVER TECHNOLOGIES
- MEDICA SPA
- MEMBRANE SOLUTIONS(NANTONG)
- NX FILTRATION
- OSMOTECH
- PERMIONICS GROUP
- REPLIGEN CORPORATION
- SYNDER FILTRATION, INC.
- THEWAY MEMBRANES
- UNISOL MEMBRANE TECHNOLOGY
- XINNA
제15장 조사 방법
제16장 부록
LSH 26.01.22The medical membranes market is projected to reach USD 6.66 billion by 2030 from USD 4.31 billion in 2025, at a CAGR of 9.1% during the forecast period. The global medical membranes market is driven by the increasing number of chronic kidney disease and diabetes patients. Continuous innovations in membrane technology like better biocompatibility, antifouling coatings, and nanofiltration capabilities are making drug purification, bioprocessing, and artificial organ applications possible.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2022-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million) Volume (Square Meters) |
| Segments | Material, Process Technology, Application, and Region |
| Regions covered | North America, Europe, Asia Pacific, South America, and Middle East & Africa |
Additionally, the increasing number of surgical procedures, preference for advanced wound dressings, and tissue engineering scaffolds are also supporting market growth. Meanwhile, there is strong investment in single-use disposable membrane systems in biotech and pharmaceutical manufacturing for eliminating cross-contamination. Moreover, the healthcare infrastructure in emerging markets (especially Asia Pacific), the aging population in mature regions, and significant R&D on bio-artificial organs and regenerative medicine are all contributing to the market growth.
"Polyvinylidene fluoride (PVDF) is the fastest-growing material segment in the medical membranes market during the forecast period."
Polyvinylidene fluoride (PVDF) is the fastest-growing segment in the medical membranes market due to its excellent properties. PVDF is known for its chemical resistance, heat stability, physical strength, and low protein adherence and, thus, is widely used in the purification of liquids in pharmaceuticals and biopharmaceuticals, alongside blood filtering, drug purification, and filtration of the final products of biopharmaceuticals. Hydrophilic PVDF membranes are increasingly used in medical diagnostics and biologics production, driven by the worldwide rise in chronic diseases, the growing focus on personalized medicine, and the expanding aging population. PVDF's biocompatibility is a key factor for innovations in tissue engineering, wound dressings, and virus removal, which makes it superior to competitors such as PTFE or PESU. At the same time, the pandemic-induced surge in pharmaceutical manufacturing and increased investments in advanced filtration technologies also favor PVDF.
"Ultrafiltration is projected to be the largest process technology segment in the medical membranes market during the forecast period."
Ultrafiltration (UF) is the largest technology segment in the overall medical membranes market due to its essential application in hemodialysis. Hemodialysis overcomes the limits of kidney function in more than 3 million end-stage renal disease patients, curing them by highly efficient removal of ureotoxins and keeping essential proteins in the body. UF membranes are often made from polysulfone, polyethersulfone, or modified PES, and they have precise corresponding molecular weight cut-offs (5-50kDa) that give excellent biocompatibility as well as high flux rates and superior hemocompatibility. Thus, they have become the standard for purification in dialysis clinics and ICUs. The strong growth of the medical membranes market is also supported by the expanding use of ultrafiltration (UF) beyond renal therapy. UF is widely applied in microbiology and pharmaceutical downstream processing for concentrating, diafiltering, and exchanging buffers in monoclonal antibodies, vaccines, and recombinant proteins, all of which are increasing rapidly with the rise of biologics. Its ability to pump at low transmembrane pressure and usage of advanced surface modifications to reduce fouling make it even more preferred over microfiltration, reverse osmosis, or nanofiltration in high-volume, high-value medical applications.
"Europe is projected to be the second-largest medical membranes market during the forecast period."
Europe is the second-largest medical membranes market. The market in the region is driven by its excellent healthcare systems, high spending on renal care per capita, and a large number of elderly people with a high prevalence of chronic kidney diseases. The market in the region is also supported by the presence of major membrane manufacturers with significant R&D and production capacity in Germany, France, Italy, and Switzerland. Stringent EU regulatory standards ensure that only the best products are delivered to the market. However, this creates barriers for new players. Moreover, widespread reimbursement for dialysis treatments, the growing use of single-use bioprocessing systems in the fast-expanding biologics sector, and significant public and private investments in fields like regenerative medicine and bioartificial organs contribute to the demand for the product.
Extensive primary interviews were conducted to determine and verify the market size for several segments and subsegments and the information gathered through secondary research.
The breakdown of primary interviews is given below:
- By Department: Tier 1 - 40%, Tier 2 - 25%, and Tier 3 - 35%
- By Designation: C Level - 35%, Director Level - 30%, and Executives - 35%
- By Region: North America - 25%, Europe - 45%, Asia Pacific - 20%, South America - 5%, and Middle East & Africa - 5%
Asahi Kasei Corporation (Japan), Mann+Hummel (Germany), Sartorius AG (Germany), Merck KGaA (Germany), Solventum (US), Cytiva (US), W. L. Gore & Associates, Inc. (US), Kovalus Separation Solutions (US),and Cobetter (China), among others, are some of the key players in the medical membranes market. The study includes an in-depth competitive analysis of these key players in the medical membranes market, with their company profiles, recent developments, and key market strategies.
Research Coverage
The market study covers the medical membranes market across various segments. It aims to estimate the market size and the growth potential of this market across different segments based on material, process technology, application, and region. The study also includes an in-depth competitive analysis of key players in the market, their company profiles, key observations related to their products and business offerings, recent developments undertaken by them, and key growth strategies adopted by them to improve their position in the medical membranes market.
Key Benefits of Buying the Report
The report is expected to help the market leaders/new entrants in this market with the closest approximations of the revenue numbers of the overall medical membranes market and its segments and subsegments. This report is projected to help stakeholders understand the competitive landscape of the market, gain insights to improve the position of their businesses, and plan suitable go-to-market strategies. The report also aims to help stakeholders understand the pulse of the market and provide them with information on the key market drivers, restraints, challenges, and opportunities.
The report provides insights into the following pointers:
- Analysis of key drivers (Rising prevalence of chronic diseases, Growing adoption of membrane-based separation technologies, Increasing number of surgical procedures), restraints (High cost of advanced medical membranes, Stringent regulatory approval processes, Risk of membrane fouling and limited lifespan of membranes), opportunities (Growing demand for single-use membrane systems in bioprocessing, Expansion in emerging markets, Integration of nanotechnology and smart membranes), challenges (Complexity in scaling up production of high-performance membranes, Environmental concerns, and Intense competition).
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product & service launches in the medical membranes market
- Market Development: Comprehensive information about lucrative markets - the report analyses the medical membranes market across varied regions.
Market Diversification: Exhaustive information about new products & services, untapped geographies, recent developments, and investments in the medical membranes market
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and service offerings of leading players like Asahi Kasei Corporation (Japan), Mann+Hummel (Germany), Sartorius AG (Germany), Merck KGaA (Germany), Solventum (US), Cytiva (US), W. L. Gore & Associates, Inc. (US), Kovalus Separation Solutions (US), and Cobetter (China), among others
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKETS COVERED AND REGIONAL SCOPE
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.3.5 UNIT CONSIDERED
- 1.4 STAKEHOLDERS
2 EXECUTIVE SUMMARY
3 PREMIUM INSIGHTS
- 3.1 ATTRACTIVE OPPORTUNITIES FOR PLAYERS IN MEDICAL MEMBRANES MARKET
- 3.2 MEDICAL MEMBRANES MARKET, BY APPLICATION
- 3.3 MEDICAL MEMBRANES MARKET, BY MATERIAL
- 3.4 MEDICAL MEMBRANES MARKET, BY PROCESS TECHNOLOGY
- 3.5 MEDICAL MEMBRANES MARKET, BY REGION
4 MARKET OVERVIEW
- 4.1 INTRODUCTION
- 4.2 MARKET DYNAMICS
- 4.2.1 DRIVERS
- 4.2.1.1 Rising prevalence of chronic diseases
- 4.2.1.2 Advancements in biotechnology and drug delivery systems
- 4.2.1.3 Rising demand for hemodialysis and filtration technologies
- 4.2.1.4 Expansion of healthcare infrastructure and increasing access globally
- 4.2.2 RESTRAINTS
- 4.2.2.1 High manufacturing and production costs
- 4.2.2.2 Stringent regulatory approvals and compliance
- 4.2.2.3 Membrane fouling and degradation issues
- 4.2.3 OPPORTUNITIES
- 4.2.3.1 Growing demand for single-use membrane systems in bioprocessing
- 4.2.3.2 Rapid expansion of medical membranes into emerging economies
- 4.2.4 CHALLENGES
- 4.2.4.1 Complexity in scaling up production of high-performance membranes
- 4.2.4.2 Environmental concerns
- 4.2.1 DRIVERS
- 4.3 UNMET NEEDS AND WHITE SPACES
- 4.3.1 UNMET NEEDS IN MEDICAL MEMBRANES MARKET
- 4.3.2 WHITE SPACE OPPORTUNITIES
- 4.4 INTERCONNECTED MARKETS AND CROSS-SECTOR OPPORTUNITIES
- 4.4.1 INTERCONNECTED MARKETS
- 4.4.2 CROSS-SECTOR OPPORTUNITIES
- 4.5 EMERGING BUSINESS MODELS AND ECOSYSTEM SHIFTS
- 4.5.1 EMERGING BUSINESS MODELS
- 4.5.2 ECOSYSTEM SHIFTS
- 4.6 STRATEGIC MOVES BY TIER-1/2/3 PLAYERS
- 4.6.1 KEY MOVES AND STRATEGIC FOCUS
5 INDUSTRY TRENDS
- 5.1 PORTER'S FIVE FORCES ANALYSIS
- 5.1.1 THREAT OF NEW ENTRANTS
- 5.1.2 THREAT OF SUBSTITUTES
- 5.1.3 BARGAINING POWER OF SUPPLIERS
- 5.1.4 BARGAINING POWER OF BUYERS
- 5.1.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.2 MACROECONOMIC INDICATORS
- 5.2.1 GLOBAL GDP TRENDS
- 5.3 VALUE CHAIN ANALYSIS
- 5.4 ECOSYSTEM ANALYSIS
- 5.5 PRICING ANALYSIS
- 5.5.1 AVERAGE SELLING PRICE TREND, BY REGION
- 5.6 TRADE ANALYSIS
- 5.6.1 IMPORT SCENARIO (HS CODE 901890)
- 5.6.2 EXPORT SCENARIO (HS CODE 901890)
- 5.7 KEY CONFERENCES AND EVENTS
- 5.8 TRENDS AND DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.9 INVESTMENT AND FUNDING SCENARIO
- 5.10 CASE STUDY ANALYSIS
- 5.10.1 ENSURING RELIABLE CLEANABILITY OF MEDICAL ULTRAFILTRATION MEMBRANES - MERCK MILLIPORE
- 5.10.2 ADVANCED MEDICAL MEMBRANE SOLUTION FOR HOSPITAL WASTEWATER TREATMENT BY IMEMFLO
- 5.11 2025 US TARIFF
- 5.11.1 INTRODUCTION
- 5.11.2 KEY TARIFF RATES
- 5.11.3 PRICE IMPACT ANALYSIS
- 5.11.4 IMPACT ON KEY REGIONS
- 5.11.4.1 North America
- 5.11.4.2 Europe
- 5.11.4.3 Asia Pacific
- 5.11.5 IMPACT ON END-USE INDUSTRIES
6 STRATEGIC DISRUPTION THROUGH TECHNOLOGY, PATENTS, DIGITAL, AND AI ADOPTIONS
- 6.1 KEY EMERGING TECHNOLOGIES
- 6.1.1 CELL MEMBRANE-COATED NANOPARTICLES (CNPS)
- 6.1.2 BIOCOMPATIBLE AND BIODEGRADABLE COMPOSITE MEMBRANES
- 6.2 COMPLEMENTARY TECHNOLOGIES
- 6.2.1 SURFACE MODIFICATION & ANTIMICROBIAL/ANTIFOULING COATINGS
- 6.3 TECHNOLOGY/PRODUCT ROADMAP
- 6.3.1 SHORT-TERM (2025-2027) | FOUNDATION & EARLY COMMERCIALIZATION
- 6.3.2 MID-TERM (2027-2030) | EXPANSION & STANDARDIZATION
- 6.3.3 LONG-TERM (2030-2035+) | MASS COMMERCIALIZATION & DISRUPTION
- 6.4 PATENT ANALYSIS
- 6.4.1 INTRODUCTION
- 6.4.2 METHODOLOGY
- 6.4.3 DOCUMENT TYPE
- 6.4.4 INSIGHTS
- 6.4.5 LEGAL STATUS OF PATENTS
- 6.4.6 JURISDICTION ANALYSIS
- 6.4.7 TOP APPLICANTS
- 6.4.8 TOP 10 PATENT OWNERS IN LAST 10 YEARS
- 6.5 FUTURE APPLICATIONS
- 6.5.1 NEXT-GENERATION HEMODIALYSIS & ARTIFICIAL ORGANS
- 6.5.2 ADVANCED WOUND-CARE & TISSUE-ENGINEERING MEMBRANES
- 6.5.3 CONTROLLED DRUG-DELIVERY & IMPLANTABLE THERAPEUTIC MEMBRANES
- 6.5.4 POINT-OF-CARE DIAGNOSTICS & MICROFLUIDIC LAB-ON-CHIP DEVICES
- 6.5.5 WEARABLE HEALTH SENSORS & CONTINUOUS BIOMARKER MONITORING
- 6.6 IMPACT OF AI/GEN AI ON MEDICAL MEMBRANES MARKET
- 6.6.1 TOP USE CASES AND MARKET POTENTIAL
- 6.6.2 BEST PRACTICES IN MEDICAL MEMBRANES PROCESSING
- 6.6.3 CASE STUDIES OF AI IMPLEMENTATION IN MEDICAL MEMBRANES MARKET
- 6.6.4 INTERCONNECTED ADJACENT ECOSYSTEM AND IMPACT ON MARKET PLAYERS
- 6.6.5 CLIENTS' READINESS TO ADOPT GENERATIVE AI IN MEDICAL MEMBRANES MARKET
- 6.7 SUCCESS STORIES AND REAL-WORLD APPLICATIONS
- 6.7.1 PALL CORPORATION: HEMODIALYSIS MEMBRANES
- 6.7.2 MERCK KGAA: BIOFUNCTIONAL MEMBRANES
- 6.7.3 BAXTER INTERNATIONAL: EXTRACORPOREAL MEMBRANES
7 SUSTAINABILITY AND REGULATORY LANDSCAPE
- 7.1 REGIONAL REGULATIONS AND COMPLIANCE
- 7.1.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 7.1.2 INDUSTRY STANDARDS
- 7.2 SUSTAINABILITY INITIATIVES
- 7.3 SUSTAINABILITY IMPACT AND REGULATORY POLICY INITIATIVES
- 7.4 CERTIFICATIONS, LABELING, AND ECO-STANDARDS
8 CUSTOMER LANDSCAPE & BUYER BEHAVIOR
- 8.1 DECISION-MAKING PROCESS
- 8.2 KEY STAKEHOLDERS AND BUYING CRITERIA
- 8.2.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 8.2.2 BUYING CRITERIA
- 8.3 ADOPTION BARRIERS & INTERNAL CHALLENGES
- 8.4 UNMET NEEDS IN VARIOUS APPLICATIONS
- 8.5 MARKET PROFITABILITY
- 8.5.1 REVENUE POTENTIAL
- 8.5.2 COST DYNAMICS
- 8.5.3 MARGIN OPPORTUNITIES IN KEY END-USE INDUSTRIES
9 MEDICAL MEMBRANES MARKET, BY MATERIAL
- 9.1 INTRODUCTION
- 9.2 POLYSULFONE (PSU) & POLYETHERSULFONE (PESU)
- 9.2.1 ADVANCING DIALYSIS AND CLINICAL FILTRATION WITH BIOCOMPATIBLE, HIGH-FLUX MEMBRANE TECHNOLOGIES TO DRIVE MARKET
- 9.3 POLYVINYLIDENE FLUORIDE (PVDF)
- 9.3.1 EXCELLENT MECHANICAL STRENGTH, THERMAL STABILITY, AND CHEMICAL RESISTANCE TO DRIVE MARKET
- 9.3.2 HYDROPHOBIC PVDF MEMBRANE
- 9.3.3 HYDROPHOBIC PVDF MEMBRANE
- 9.4 POLYTETRAFLUOROETHYLENE (PTFE)
- 9.4.1 ADVANCING MEDICAL VENT FILTRATION WITH ULTRA-RESILIENT MEMBRANE MATERIALS TO DRIVE MARKET
- 9.5 POLYPROPYLENE (PP)
- 9.5.1 OPTIMIZING THERMAL STABILITY FOR HIGH-TEMPERATURE USE TO DRIVE MARKET
- 9.6 POLYACRYLONITRILE (PAN)
- 9.6.1 PROVIDES SUPERIOR CHEMICAL RESISTANCE IN AGGRESSIVE DRUG FILTRATION TO DRIVE MARKET
- 9.7 POLYAMIDE (PA)
- 9.7.1 ENABLES HIGH PERMSELECTIVITY IN PHARMACEUTICAL NANOFILTRATION AND DRUG CONCENTRATION TO DRIVE MARKET
- 9.8 MODIFIED ACRYLICS
- 9.8.1 DELIVERS UNMATCHED OPTICAL CLARITY FOR HIGH-SENSITIVITY DIAGNOSTIC DEVICES TO DRIVE MARKET
- 9.9 OTHER MATERIALS
10 MEDICAL MEMBRANES MARKET, BY PROCESS TECHNOLOGY
- 10.1 INTRODUCTION
- 10.2 ULTRAFILTRATION
- 10.2.1 TRANSFORMING MEDICAL PURIFICATION WORKFLOWS WITH HIGH-PERFORMANCE MEMBRANE SOLUTIONS TO DRIVE MARKET
- 10.3 NANOFILTRATION
- 10.3.1 REMOVES ENDOTOXINS, HORMONE RESIDUES, ANTIBIOTICS, AND OTHER TRACES FROM PROCESS WATER AND MEDICAL SOLUTIONS TO DRIVE MARKET
- 10.4 MICROFILTRATION
- 10.4.1 RISING DEMAND FOR ENERGY-EFFICIENT AIR CONDITIONING SYSTEMS AND RETROFITTING OF OLDER BUILDINGS TO DRIVE MARKET
- 10.5 OTHER PROCESS TECHNOLOGIES
11 MEDICAL MEMBRANES MARKET, BY APPLICATION
- 11.1 INTRODUCTION
- 11.2 PHARMACEUTICAL FILTRATION
- 11.2.1 ENHANCING BIOPHARMACEUTICAL SAFETY WITH HIGH-PERFORMANCE MEMBRANE SOLUTIONS TO DRIVE MARKET
- 11.3 HEMODIALYSIS
- 11.3.1 EMPOWERING RENAL SUPPORT WITH PRECISION FILTRATION TO DRIVE MARKET
- 11.4 DRUG DELIVERY
- 11.4.1 ENHANCING TREATMENT RELIABILITY THROUGH CONTROLLED DIFFUSION TECHNOLOGY TO DRIVE MARKET
- 11.5 IV FUSION & STERILE FILTRATION
- 11.5.1 ADVANCING CLINICAL OUTCOMES THROUGH CUTTING-EDGE FILTRATION AND DELIVERY TO DRIVE MARKET
- 11.6 MEMBRANE OXYGENATOR
- 11.6.1 DRIVING EFFICIENT AND SAFE OXYGEN DELIVERY WITH MEMBRANE INNOVATION TO DRIVE MARKET
- 11.7 APHERESIS MEMBRANE
- 11.7.1 TRANSFORMING BLOOD TREATMENT THROUGH ADVANCED MEMBRANE TECHNOLOGY TO DRIVE MARKET
- 11.8 OTHER APPLICATIONS
12 MEDICAL MEMBRANES MARKET, BY REGION
- 12.1 INTRODUCTION
- 12.2 ASIA PACIFIC
- 12.2.1 CHINA
- 12.2.1.1 Accelerating expansion in healthcare filtration technologies to drive market
- 12.2.2 INDIA
- 12.2.2.1 Seizing opportunities in cost-effective dialysis and filtration to drive market
- 12.2.3 JAPAN
- 12.2.3.1 Strengthening healthcare outcomes with high-tech filtration to drive market
- 12.2.4 SOUTH KOREA
- 12.2.4.1 Rising demand from healthcare, pharmaceutical, and biotechnology sector to drive market
- 12.2.5 REST OF ASIA PACIFIC
- 12.2.1 CHINA
- 12.3 NORTH AMERICA
- 12.3.1 US
- 12.3.1.1 Surge in construction activities to drive market
- 12.3.2 CANADA
- 12.3.2.1 Rise of new housing projects to drive market
- 12.3.3 MEXICO
- 12.3.3.1 Expanding industrial capabilities and healthcare systems to drive market
- 12.3.1 US
- 12.4 EUROPE
- 12.4.1 GERMANY
- 12.4.1.1 Surge in construction of residential infrastructure to drive market
- 12.4.2 UK
- 12.4.2.1 Rising demand for hemodialysis, pharmaceutical filtration, and IV infusion systems to drive market
- 12.4.3 FRANCE
- 12.4.3.1 Policy and financial incentives for building renovation and low-carbon heating to drive market
- 12.4.4 ITALY
- 12.4.4.1 Investments in residential buildings and renovation activities to drive market
- 12.4.5 SPAIN
- 12.4.5.1 High demand for commercial air conditioning units to drive market
- 12.4.6 REST OF EUROPE
- 12.4.1 GERMANY
- 12.5 MIDDLE EAST & AFRICA
- 12.5.1 SAUDI ARABIA
- 12.5.1.1 Trend of small and affordable housing units to drive market
- 12.5.2 UAE
- 12.5.2.1 Projects aimed at promoting economic development to drive market
- 12.5.3 SOUTH AFRICA
- 12.5.3.1 Regional copper trade expansion to drive market
- 12.5.4 REST OF MIDDLE EAST & AFRICA
- 12.5.1 SAUDI ARABIA
- 12.6 SOUTH AMERICA
- 12.6.1 BRAZIL
- 12.6.1.1 Rapid industrial growth to drive market
- 12.6.2 ARGENTINA
- 12.6.2.1 Economic stabilization to drive market
- 12.6.3 REST OF SOUTH AMERICA
- 12.6.1 BRAZIL
13 COMPETITIVE LANDSCAPE
- 13.1 INTRODUCTION
- 13.2 KEY PLAYER STRATEGIES/RIGHT TO WIN, 2023-2025
- 13.3 REVENUE ANALYSIS, 2022-2024
- 13.4 MARKET SHARE ANALYSIS, 2024
- 13.5 COMPANY VALUATION AND FINANCIAL METRICS
- 13.6 PRODUCT COMPARISON
- 13.7 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 13.7.1 STARS
- 13.7.2 EMERGING LEADERS
- 13.7.3 PERVASIVE PLAYERS
- 13.7.4 PARTICIPANTS
- 13.7.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 13.7.5.1 Company footprint
- 13.7.5.2 Region footprint
- 13.7.5.3 Material footprint
- 13.7.5.4 Process technology footprint
- 13.7.5.5 Application footprint
- 13.8 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 13.8.1 PROGRESSIVE COMPANIES
- 13.8.2 RESPONSIVE COMPANIES
- 13.8.3 DYNAMIC COMPANIES
- 13.8.4 STARTING BLOCKS
- 13.8.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 13.8.5.1 Detailed list of key startups/SMEs
- 13.8.5.2 Competitive benchmarking of startups/SMEs
- 13.9 COMPETITIVE SCENARIO
- 13.9.1 DEALS
- 13.9.2 EXPANSIONS
14 COMPANY PROFILES
- 14.1 KEY PLAYERS
- 14.1.1 ASAHI KASEI CORPORATION
- 14.1.1.1 Business overview
- 14.1.1.2 Products offered
- 14.1.1.3 MnM view
- 14.1.1.3.1 Right to win
- 14.1.1.3.2 Strategic choices
- 14.1.1.3.3 Weaknesses and competitive threats
- 14.1.2 MANN+HUMMEL
- 14.1.2.1 Business overview
- 14.1.2.2 Products offered
- 14.1.2.3 MnM view
- 14.1.2.3.1 Right to win
- 14.1.2.3.2 Strategic choices
- 14.1.2.3.3 Weaknesses and competitive threats
- 14.1.3 SARTORIUS AG
- 14.1.3.1 Business overview
- 14.1.3.2 Products offered
- 14.1.3.3 MnM view
- 14.1.3.3.1 Right to win
- 14.1.3.3.2 Strategic choices
- 14.1.3.3.3 Weaknesses and competitive threats
- 14.1.4 MERCK KGAA
- 14.1.4.1 Business overview
- 14.1.4.2 Products offered
- 14.1.4.3 Recent developments
- 14.1.4.3.1 Expansions
- 14.1.4.4 MnM view
- 14.1.4.4.1 Right to win
- 14.1.4.4.2 Strategic choices
- 14.1.4.4.3 Weaknesses and competitive threats
- 14.1.5 SOLVENTUM
- 14.1.5.1 Business overview
- 14.1.5.2 Products offered
- 14.1.5.3 Recent developments
- 14.1.5.3.1 Deals
- 14.1.5.4 MnM view
- 14.1.5.4.1 Right to win
- 14.1.5.4.2 Strategic choices
- 14.1.5.4.3 Weaknesses and competitive threats
- 14.1.6 CYTIVA (DANAHER CORPORATION)
- 14.1.6.1 Business overview
- 14.1.6.2 Products offered
- 14.1.6.3 Recent developments
- 14.1.6.3.1 Deals
- 14.1.6.3.2 Expansions
- 14.1.6.4 MnM view
- 14.1.7 W. L. GORE & ASSOCIATES, INC.
- 14.1.7.1 Business overview
- 14.1.7.2 Products offered
- 14.1.7.3 MnM view
- 14.1.8 KOVALUS SEPARATION SOLUTIONS
- 14.1.8.1 Business overview
- 14.1.8.2 Products offered
- 14.1.8.3 MnM view
- 14.1.9 COBETTER
- 14.1.9.1 Business overview
- 14.1.9.2 Products offered
- 14.1.9.3 MnM view
- 14.1.10 POREX
- 14.1.10.1 Business overview
- 14.1.10.2 Products offered
- 14.1.10.3 MnM view
- 14.1.1 ASAHI KASEI CORPORATION
- 14.2 OTHER PLAYERS
- 14.2.1 ADVANCED MICRODEVICES PVT. LTD.
- 14.2.2 AMERICAN MEMBRANE CORPORATION
- 14.2.3 AMS MEMTECH TECHNOLOGY (ZHEJIANG) CO., LTD
- 14.2.4 APPLIED MEMBRANE TECHNOLOGY, INC.
- 14.2.5 GRAVER TECHNOLOGIES
- 14.2.6 MEDICA SPA
- 14.2.7 MEMBRANE SOLUTIONS (NANTONG)
- 14.2.8 NX FILTRATION
- 14.2.9 OSMOTECH
- 14.2.10 PERMIONICS GROUP
- 14.2.11 REPLIGEN CORPORATION
- 14.2.12 SYNDER FILTRATION, INC.
- 14.2.13 THEWAY MEMBRANES
- 14.2.14 UNISOL MEMBRANE TECHNOLOGY
- 14.2.15 XINNA
15 RESEARCH METHODOLOGY
- 15.1 RESEARCH DATA
- 15.1.1 SECONDARY DATA
- 15.1.1.1 List of secondary sources
- 15.1.1.2 Key data from secondary sources
- 15.1.2 PRIMARY DATA
- 15.1.2.1 List of primary interview participants-demand and supply side
- 15.1.2.2 Key data from primary sources
- 15.1.2.3 Breakdown of primary interviews
- 15.1.2.4 Insights from industry experts
- 15.1.1 SECONDARY DATA
- 15.2 MARKET SIZE ESTIMATION
- 15.2.1 BOTTOM-UP APPROACH
- 15.2.2 TOP-DOWN APPROACH
- 15.3 FORECAST NUMBER CALCULATION
- 15.4 DATA TRIANGULATION
- 15.5 FACTOR ANALYSIS
- 15.6 RESEARCH ASSUMPTIONS
- 15.7 RESEARCH LIMITATIONS AND RISK ASSESSMENT
16 APPENDIX
- 16.1 DISCUSSION GUIDE
- 16.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 16.3 CUSTOMIZATION OPTIONS
- 16.4 RELATED REPORTS
- 16.5 AUTHOR DETAILS















