
|
시장보고서
상품코드
1851108
보툴리눔툭신(보톡스) : 시장 점유율 분석, 산업 동향, 통계, 성장 예측(2025-2030년)Botulinum Toxin - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
보툴리눔툭신(보톡스) 시장은 2025년에 97억 7,000만 달러를 창출하고, 2030년에는 151억 달러에 이르며, CAGR 9.11%를 나타낼 것으로 예측됩니다.

치료의 다양성이 미용 용도 이외 수요를 확대하고, 장시간 작용형 혈청형, 정밀 투여 장치, 새로운 정신과 적응증이 성장의 템포를 설정하고 있습니다. 남성 소비자층의 확대, 아시아태평양의 의료 인가 증가, 기술에 의한 투여 정밀도의 향상이 상업적인 기세를 강화하고 있습니다. 2024년 크라운 랩토리즈가 9억 2,400만 달러에 레반스 세라퓨틱스를 인수하는 등 전략적인수가 경쟁을 격화시키는 한편, 투여 기간 주도의 제품 부문을 재정의했습니다. 모방품에 대한 규제상 경계는 북미와 유럽에서의 상환의 변화와 함께 단기적인 마찰을 낳는 동시에 안전한 공급망과 트레이서블한 포장에 대한 투자에 박차를 가하고 있습니다.
세계의 보툴리눔툭신(보톡스) 시장 동향과 인사이트
미용 시술에 대한 수요 증가
2024년에는 30만 명 이상의 남성이 주사를 선택하고 남성 이용이 증가했습니다. 근육량이 증가함에 따라 복용량이 증가하면 환자 1인당 매출이 증가합니다. 25-35세의 예방치료는 평생치료 사이클을 연장하고 장기적인 매출을 높입니다. 전통적으로 보수적인 중동 시장에서도 도입이 가속화되어 문화적 장벽이 빠르게 붕괴되고 있음을 보여줍니다. 이러한 요인이 함께 거시경제의 변동을 극복할 수 있는 인구동태적으로 다양한 수요가 창출되고 있습니다.
치료 용도 확대를 위한 R&D 성장
3상 임상시험은 안면 피드백 메커니즘을 통한 우울증과 불안증에 대한 효능을 보여줍니다. 심장 수술 연구는 수술 후 심방 세동에 대한 가능성을 보여 주며 수십억 달러 규모의 병원 수익원을 시사합니다. 신경 영역의 파이프라인은 파킨슨병과 3차 신경통을 대상으로 하며, 소아과 영역에서는 뇌성 마비의 승인을 얻어 재발 주도형 매출이 확대됩니다. 유전자 마커를 활용한 Precision Medicine 접근법은 더 높은 효능과 더 적은 부작용을 약속하고 의사의 신뢰와 지불자의 지원을 강화하고 있습니다.
부작용과 안전성에 대한 우려
규제 당국은 미국 9개 주에 걸쳐 위조 수입품과 관련된 17건의 유해 사건을 조사했으며, 개업의 경계를 강화하고 컴플라이언스 비용을 밀어 올리고 있습니다. FDA의 단속은 공급망의 취약성을 돋보이게 하고, 부정 개봉 방지 포장이나 직렬화의 보급을 촉진하고 있습니다. 드물지만, 중화 항체에 의해 만성 치료제의 장기적인 효능이 저하되기 때문에 연구개발은 혈청형의 다양화를 목표로 하고 있습니다. 드물게 독소가 확산되는 사건이 발생하면 의료 과오의 보험료가 증가하고 소규모 진료소의 참여 의욕을 줄일 수 있습니다.
부문 분석
Type A는 2024년 보툴리눔툭신(보톡스) 시장 점유율의 91.67%를 기록한 긴 안전성 실적과 광범위한 적응증을 반영했습니다. B형은 틈새이면서 CAGR 9.83%로 확대되고 있어 항체 양성 환자나 경축증례에 대응하고 있습니다. 닥사이파이와 같은 투여 기간 연장형 제형은 전통적인 12주 사이클을 덮고 중앙값에서 24주 결과를 제공합니다. 현재 임상시험 중의 액제 후보는 재구성 불필요한 취급과 보존성의 향상을 약속하고 있습니다. 한국 발상의 Letybo가 미국에서 승인되어 보톡스의 브랜드 에퀴티에 과제하고 있기 때문에 경쟁의 격렬함이 증가하고 있습니다. 제품을 둘러싼 환경은 환자의 편의성과 클리닉의 효율성을 중시한 지속시간과 제제 기반의 범주로 이행하고 있습니다.
유형 A는 여전히 연구 자금과 의사의 선호도의 대부분을 차지하고 있지만, 특허가 해지되면 지불자는 비용면에서 유리한 바이오 시밀러에 끌릴 수 있습니다. B형 공급업체는 자율신경질환을 대상으로 하는 전달을 모색하고 임상폭을 넓히고 있습니다. 혈청형과 제형의 유형가 늘어나고 있어 의료기관에 대해서는 재고 결정이 복잡해지고 있지만, 환자에게는 치료 빈도, 발병 속도, 항체 프로파일에 맞추어 커스터마이즈된 선택사항을 제공하게 됩니다.
미용 주사는 2024년에 62.39%의 매출을 유지했지만, 신경학적, 심장학적, 정신의학적 효과가 입증됨에 따라 치료 절차는 10.66%의 연평균 복합 성장률(CAGR)로 급성장하고 있습니다. 만성 편두통의 예방은 12주 사이클의 지속적인 효능으로 인해 여전히 수익이 필요합니다. 그래블러 주사를 이용한 우울증 프로토콜은 44.8%의 연주 효율을 얻었고, 정신과 수익의 가능성을 강조했습니다. 심장 수술의 임상시험은 병원의 심장 수술실에서 신경독을 사용하여 수술 후 심방 세동이 감소한 것으로보고되었습니다.
치료 용도의 보툴리눔툭신(보톡스) 시장 규모는 규제 당국이 적응증을 추가로 승인함에 따라 급증할 것으로 예측됩니다. 상지 경련에 대한 캐나다 보건부의 투여 상한 인상은 임상 신뢰 증가를 나타냅니다. 의료 용도에서는 보험 적용이 강화되고, 예측 가능한 청구의 흐름이 형성되어, 선택적 미용 목적의 주기성을 상쇄합니다. 전반적으로 치료법의 확대로 안정적인 상환을 받을 수 있는 의료상 필요한 분야로 판매 포트폴리오의 밸런스가 조정되고 있습니다.
지역 분석
북미는 상환경로 정착과 숙련된 주사기 네트워크로 2024년 매출 점유율은 43.27%를 유지했습니다. FDA의 감시가 위조품의 침투를 억제하고 높은 평균 판매 가격을 지원하고 있지만, 2024년 단속 사례에서 그레이마켓 리스크가 뿌리 깊게 남아 있는 것으로 밝혀졌습니다. 메디케어에 의한 치료제 적용의 재검토는 화장품의 상환이 제한된 채라도 추가 성장을 초래할 수 있습니다.
CAGR 11.75%를 나타낼 것으로 예측되는 아시아태평양은 중국 국가 의약품국(National Medical Products Administration)의 승인과 중간층 확대가 견인하여 가장 빠른 속도로 성장하고 있습니다. 구미의 제조업체는 현지의 파트너와 판매 제휴를 맺고, 지역의 입찰 제도를 이용하고 있습니다. 제조 비용 절감과 규제 당국의 적극적인 참여에 힘입은 한국의 혁신자들은 일본, 태국, 미국으로 진출해 경쟁 역학을 변화시키고 있습니다.
유럽은 새로운 적응증을 추진하는 범 EU 임상시험 네트워크에 의해 지원되고 꾸준하지만 완만 한 확대를 보여줍니다. CE 인증의 조화는 장치 지원 전달 시스템 시장 출시 시간을 단축합니다. 라틴아메리카와 중동 및 아프리카는 바이오시밀러 승인과 현지화된 충전 마감 공장이 접근을 뒷받침하고 있는 신흥 프론티어입니다. Daewoong은 UAE에, Meditox는 브라질로, 제조 거점의 산업적 다양화는 지역의 자급자족과 공급망의 강인화를 추진하고 있다는 것을 뒷받침하고 있습니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 서론
- 조사 전제조건과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 상황
- 시장 개요
- 시장 성장 촉진요인
- 미용시술에 대한 수요가 높아짐
- 적응 확대를 위한 연구개발 성장
- 정밀 투여 주사 장치는 낭비를 생략
- 2단계에 들어간 정신질환 적응증
- 지속시간이 긴 신경독의 상업화
- 신흥 시장에서의 바이오시밀러 A형의 승인
- 시장 성장 억제요인
- 부작용과 안전성에 대한 우려
- 한정적인 미용 시술의 보험 상환
- 위조·불법 주사약의 단속을 촉진
- 중화 항체에 의한 장기 효능의 저하
- 가치/공급망 분석
- 규제 상황
- 기술 전망
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업 간 경쟁 관계
제5장 시장 규모와 성장 예측
- 제품별
- 보툴리눔툭신(보톡스) 유형 A
- 보툴리눔툭신(보톡스) 유형 B
- 용도별
- 미용 용도
- 미간 주름
- 외안각 주름
- 이마 주름
- 저작근 비대
- 입술 뒤집힘 및 잇몸 노출 미소
- 치료 용도
- 근긴장 이상증
- 만성 편두통
- 경련성 경직
- 과활동 방광
- 타액 과다 분비
- 안과 질환
- 위식도 질환(식도 이완 불능증)
- 정신 질환(우울증, 불안)
- 미용 용도
- 성별
- 남성
- 여성
- 최종 사용자별
- 스파 및 뷰티센터
- 피부과 및 미용 클리닉
- 병원 및 전문 센터
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 시장 집중도
- 시장 점유율 분석
- 기업 프로파일
- AbbVie
- Ipsen
- Merz Pharma
- Galderma
- Revance Therapeutics
- Evolus
- Medytox
- Daewoong Pharmaceutical
- Hugel
- Huons Global
- Supernus Pharmaceuticals
- Lanzhou Institute of Biological Products
- Chong Kun Dang Pharma
- Alphaeon
- Ipsen Biopharm Ltd.
- Huadong Medicine
- Suneva Medical
- Croma-Pharma
제7장 시장 기회와 향후 전망
KTH 25.11.20The botulinum toxin market generated USD 9.77 billion in 2025 and is forecast to reach USD 15.10 billion by 2030, advancing at a 9.11% CAGR.
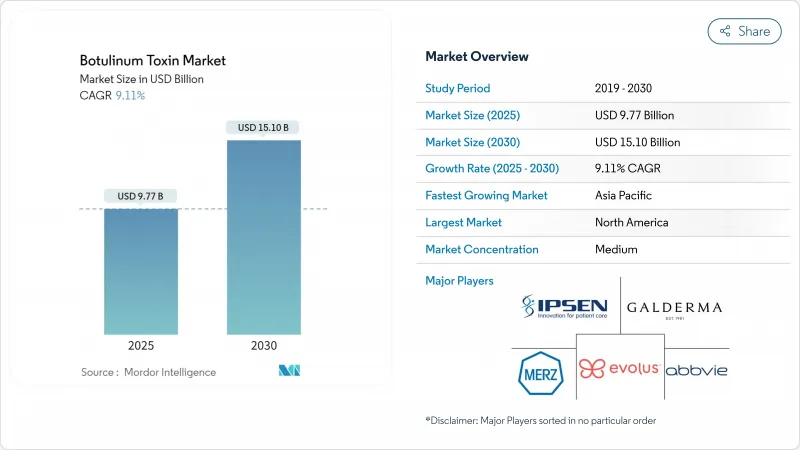
Therapeutic versatility is widening demand beyond cosmetic use, with long-acting serotypes, precision-dosing devices, and new psychiatric indications setting the growth tempo. An expanding male consumer base, rising medical approvals in Asia-Pacific, and technology-enabled dosing accuracy are reinforcing commercial momentum. Strategic acquisitions, such as Crown Laboratories' buyout of Revance Therapeutics for USD 924 million in 2024, are intensifying competition while redefining duration-led product segments. Regulatory vigilance against counterfeit products, coupled with reimbursement shifts in North America and Europe, creates near-term friction yet also spurs investment in secure supply chains and traceable packaging.
Global Botulinum Toxin Market Trends and Insights
Increasing Demand for Aesthetic Procedures
Male uptake is rising as more than 300,000 men opted for injections in 2024, reflecting cultural shifts toward routine grooming. Higher dosing requirements linked to greater muscle mass enlarge per-patient revenue. Preventative treatments in the 25-35 age cohort extend lifetime treatment cycles and heighten long-term sales. Adoption is accelerating in traditionally conservative Middle Eastern markets, indicating quickly eroding cultural barriers. Together, these factors create resilient, demographically diverse demand able to weather macro-economic swings.
Growing R&D for Expanded Therapeutic Uses
Phase III trials show efficacy in depression and anxiety via the facial-feedback mechanism. Cardiac surgery studies demonstrate potential in post-operative atrial fibrillation, hinting at multi-billion-dollar hospital revenue streams. Neurological pipeline programs target Parkinson's disease and trigeminal neuralgia, while pediatric approvals for cerebral palsy broaden recurrence-driven sales. Precision-medicine approaches leveraging genetic markers promise higher efficacy and fewer side effects, reinforcing physician confidence and payer support.
Adverse Effects & Safety Concerns
Regulators are investigating 17 adverse events linked to counterfeit imports across nine U.S. states, heightening practitioner vigilance and driving up compliance costs. FDA crackdowns underscore supply-chain vulnerabilities, prompting wider adoption of tamper-evident packaging and serialization. Neutralizing antibodies, although infrequent, reduce long-term efficacy for chronic therapies, nudging R&D toward serotype diversity. Rare toxin spread events amplify malpractice premiums and may discourage small-practice participation.
Other drivers and restraints analyzed in the detailed report include:
- Precision-Dosing Injection Devices Cut Wastage
- Psychiatric-Disorder Trials Entering Phase II
- Limited Reimbursement of Cosmetic Procedures
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Type A recorded a 91.67% botulinum toxin market share in 2024, reflecting its long safety record and broad indication roster. Type B, though niche, is expanding at 9.83% CAGR, catering to antibody-positive patients and spasticity cases. Extended-duration formulations such as Daxxify deliver median results of 24 weeks, upending the traditional 12-week cycle. Liquid candidates now in trials promise reconstitution-free handling and improved shelf life. Competitive intensity is rising as Korean-origin Letybo gains U.S. approval, challenging Botox's brand equity. The product landscape is moving toward duration- and formulation-based categories that emphasize patient convenience and clinic efficiency.
Type A still anchors most research funding and physician preference, yet payers may gravitate to cost-advantaged biosimilars once patents lapse. Type B suppliers are exploring targeted delivery to autonomic disorders, broadening their clinical narrative. The growing array of serotypes and formulations complicates stocking decisions for providers but offers patients customized options tailored to treatment frequency, onset speed, and antibody profile.
Cosmetic injections retained 62.39% revenue in 2024, but therapeutic procedures are growing faster at 10.66% CAGR as evidence mounts for neurologic, cardiac, and psychiatric benefits. Chronic migraine prevention remains a revenue cornerstone thanks to durable efficacy over multiple 12-week cycles. Depression protocols using glabellar injections yielded 44.8% response rates, underscoring psychiatry's revenue potential. Cardiac surgery trials report reduced postoperative atrial fibrillation episodes, positioning neurotoxins within hospital cardiac suites.
The botulinum toxin market size for therapeutic applications is projected to climb sharply as regulators approve additional indications. Health Canada's higher dosing ceiling for upper-limb spasticity illustrates growing clinical confidence . Insurance coverage is stronger for medical uses, creating predictable billing streams that offset elective-cosmetic cyclicality. Overall, therapeutic expansion is rebalancing sales portfolios toward medically necessary segments with stable reimbursement.
The Botulinum Toxin Market Report Segments the Industry Into by Product (Botulinum Toxin Type A, Botulinum Toxin Type B), by Application (Cosmetic Applications, Therapeutic Applications), by Gender (Male and Female) by End User (Spas and Beauty Centers, Clinics and Hospitals, and More), and Geography. The Market Sizes and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America preserved 43.27% revenue share in 2024 due to entrenched reimbursement pathways and seasoned injector networks. FDA oversight curtails counterfeit penetration and supports high average selling prices, yet enforcement cases in 2024 revealed persistent gray-market risks. Medicare's reconsideration of therapeutic coverage can unlock incremental growth, even as cosmetic reimbursement stays limited.
Asia-Pacific is the fastest mover, projected at 11.75% CAGR, driven by China's National Medical Products Administration approvals and an expanding middle class. Western manufacturers form distribution alliances with local partners to navigate regional tender systems. Korean innovators, propelled by lower production costs and proactive regulatory engagement, are penetrating Japan, Thailand, and the United States, shifting competitive dynamics.
Europe shows steady but slower expansion, aided by pan-EU clinical trial networks that advance new indications. Harmonized CE certification reduces time-to-market for device-aided delivery systems. Latin America and the Middle East & Africa collectively represent a rising frontier where biosimilar approvals and localized fill-finish plants are boosting access. Industrial diversification of manufacturing sites-to the UAE for Daewoong and to Brazil for Medytox-underscores the drive toward regional self-sufficiency and supply-chain resilience.
- Abbvie
- Ipsen
- Merz Pharma
- Galderma
- Revance Therapeutics
- Evolus
- Medytox
- Daewoong Pharmaceutical
- Hugel
- Huons Global
- Supernus Pharmaceuticals
- Lanzhou Institute of Biological Products
- Chong Kun Dang Pharma
- Alphaeon
- Ipsen Biopharm Ltd.
- Huadong Medicine
- Suneva Medical
- Croma-Pharma
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Demand For Aesthetic Procedures
- 4.2.2 Growing R&D For Expanded Therapeutic Indications
- 4.2.3 Precision-Dosing Injection Devices Cut Wastage
- 4.2.4 Psychiatric-Disorder Indications Entering Phase Ii
- 4.2.5 Commercialization Of Longer-Duration Neurotoxins
- 4.2.6 Biosimilar Type-A Approvals In Emerging Markets
- 4.3 Market Restraints
- 4.3.1 Adverse Effects & Safety Concerns
- 4.3.2 Limited Reimbursement Of Cosmetic Procedures
- 4.3.3 Counterfeit/Illegal Injectables Prompt Crackdowns
- 4.3.4 Neutralizing Antibodies Reduce Long-Term Efficacy
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Product
- 5.1.1 Botulinum Toxin Type A
- 5.1.2 Botulinum Toxin Type B
- 5.2 By Application
- 5.2.1 Cosmetic Applications
- 5.2.1.1 Glabellar Lines
- 5.2.1.2 Lateral Canthal Lines (Crow's Feet)
- 5.2.1.3 Forehead Lines
- 5.2.1.4 Masseter Hypertrophy
- 5.2.1.5 Lip Flip & Gummy Smile
- 5.2.2 Therapeutic Applications
- 5.2.2.1 Dystonia
- 5.2.2.2 Chronic Migraine
- 5.2.2.3 Spasticity
- 5.2.2.4 Overactive Bladder
- 5.2.2.5 Sialorrhea
- 5.2.2.6 Ophthalmologic Disorders
- 5.2.2.7 Gastro-oesophageal Disorders (Achalasia)
- 5.2.2.8 Psychiatric Disorders (Depression, Anxiety)
- 5.2.1 Cosmetic Applications
- 5.3 By Gender
- 5.3.1 Male
- 5.3.2 Female
- 5.4 By End User
- 5.4.1 Spas & Beauty Centers
- 5.4.2 Dermatology & Aesthetic Clinics
- 5.4.3 Hospitals & Specialty Centers
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 AbbVie
- 6.3.2 Ipsen
- 6.3.3 Merz Pharma
- 6.3.4 Galderma
- 6.3.5 Revance Therapeutics
- 6.3.6 Evolus
- 6.3.7 Medytox
- 6.3.8 Daewoong Pharmaceutical
- 6.3.9 Hugel
- 6.3.10 Huons Global
- 6.3.11 Supernus Pharmaceuticals
- 6.3.12 Lanzhou Institute of Biological Products
- 6.3.13 Chong Kun Dang Pharma
- 6.3.14 Alphaeon
- 6.3.15 Ipsen Biopharm Ltd.
- 6.3.16 Huadong Medicine
- 6.3.17 Suneva Medical
- 6.3.18 Croma-Pharma
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment

















