
|
시장보고서
상품코드
1849838
태아 및 신생아 케어 장비 시장 : 점유율 분석, 산업 동향, 통계, 성장 예측(2025-2030년)Fetal And Neonatal Care Equipment - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
세계의 태아 및 신생아 케어 장비 시장은 2025년 96억 8,000만 달러로, 2030년까지 131억 5,000만 달러에 이르고, CAGR 6.31%를 보일 것으로 예측됩니다.

조산률 상승, AI를 활용한 예측 분석의 보급, 신생아 집중 치료 능력을 확대하는 대규모 정부 투자의 수렴이 성장의 요인이며, 특히 중국에서는 2024년 임산부 사망률이 10만명당 14.3명까지 저하됐습니다. 무선 휴대용 모니터에 대한 수요가 증가함에 따라 수년간의 연결 시스템에 대한 의존이 해체되고 있으며, 사이버 보안 요구 사항은 제품 설계 및 조달 결정에 영향을 미칩니다. 선도적인 제조업체는 AI 알고리즘, 시큐어 바이 디자인 아키텍처 및 클라우드 연결을 통합하고 종종 태아 진단을 전문으로 하는 신흥 기업을 대상으로 인수함으로써 경쟁 역학이 치열 해지고 있습니다.
세계 태아 및 신생아 케어 장비 시장 동향 및 통찰
조산과 저체중 출산 증가
미국에서는 2023년 조산이 출생아의 10.4%에 달하고, 지난 10년간 최고를 기록했기 때문에 고급 인큐베이터, 인공호흡기, 모니터링 시스템에 대한 수요가 지속적으로 높아지고 있습니다. March of Dimes는 조산에 의한 연간 경제 손실 금액을 252억 달러로 추정하고 있으며 조기 개입 기술에 대한 병원에 대한 관심이 높아지고 있습니다. 중국의 CARE-Preterm 코호트에서는 초조산아의 사망률이 10.74%였음을 지적하고 있으며, 고급 보조장비의 임상적 필요성을 강조하고 있습니다. 40세 이상 여성의 조산률은 14.8%로 AI를 활용한 태아 진단에 돈을 지불할 준비가 된 프리미엄층이 형성되어 있습니다. 이러한 요인이 결합되어 태아 및 신생아 케어 장비 시장을 지원하는 신생아 장비 및 정기 소모품에 대한 수요가 증가하고 있습니다.
낮은 자원 환경에서 포인트 오브 케어 태아 모니터링 수요 가속
합리적인 혁신은 신흥 국가의 관리 경로를 재정의합니다. 말라위의 조종사 시험에서 56달러의 Optoco 외장형 토코다이나모미터는 고가의 시스템에 비해 임상적으로 허용되는 측정치를 보였습니다. 우간다의 Moyo 장치의 배치는 사용자 수용성을 유지하면서 분만 중 태아 심박수 검출을 개선했습니다. 비침습적인 프로토타입에 보상을 주는 NIH의 자금 지원은 저가 모니터의 상업적 스케일업을 장려하는 것입니다. 이러한 동향을 종합하면 저자원 환경에서 새로운 구매자층을 개척함으로써 태아 및 신생아 케어 장비 시장이 확대됩니다.
신규 장비 승인을 위한 엄격한 규제 일정
FDA의 신규 승인은 인원 삭감으로 심사가 지연되고 상품화 스케줄에 시간이 걸려 수익이 연기되었기 때문에 2024년 12건에 대해 2025년은 불과 2건으로 감소했습니다. 2026년 2월에 시행되는 품질 시스템 규제의 개정으로 새로운 문서화 레이어가 도입되어 컴플라이언스 비용이 상승합니다. 섹션 524B에서 의무화된 보안 문서화는 제출 서류를 더욱 증가시킵니다. 인공지능 진단의 최전선에 있는 소규모 혁신자들은 자원 제약에 직면하고 있으며, 제품 출시를 지연시키고 태아 및 신생아 케어 장비 시장의 성장을 억제할 수 있습니다.
부문 분석
신생아 의료장비는 확대하는 NICU에서 판매되는 인큐베이터, 인공 호흡기, 체온 조절 시스템에 의해 지원되고 태아 및 신생아 관리 장비 시장의 2024년 판매 중 56.23%를 차지했습니다. 인큐베이터는 습도가 조정된 마이크로 환경이나 Draeger의 BiliPredics와 같은 통합 황달 예측 소프트웨어 등의 기능 강화에 의해 임상적 가치를 높여 출하 대수를 견인했습니다. 호흡 보조 장치는 초조산기의 생존율의 상승을 돌풍으로, Vyaire의 fabian Therapy evolution은 수동 적정 시간을 단축하는 폐쇄 루프 FiO2 컨트롤을 제공하게 되었습니다. 신생아 호흡 보조 장치의 태아 및 신생아 케어 장비 시장 규모는 지침이 조기 비침습적 인공 호흡을 권장하기 때문에 2030년까지 연평균 복합 성장률(CAGR) 7.2%로 상승할 것으로 예측됩니다.
태아 케어 장비는 절대적인 매출이 작지만, CAGR 8.02%로 추이합니다. GE의 Vscan Air CL과 Exo의 Iris 핸드헬드에 탑재된 SweepAI 알고리즘으로 대표되는 AI 강화 초음파는 검출의 충실도를 향상시키면서 검사 시간을 단축합니다. RADx의 최종 전형에 남아 있는 6개 회사에 7만 5,000달러를 수여한 NIH의 자금 지원은 비침습적인 맥박산소측정과 웨어러블 심전도 솔루션에서 파이프라인의 기세를 검증하는 것입니다. 맥박 산소 측정기의 태아 및 신생아 관리 장비 시장 규모는 연간 9.4% 증가할 것으로 예상되며, 이는 분만 및 신생아 조기에 케이블이 없는 연속 산소 포화도 추적에 대한 수요를 반영합니다.
지역 분석
북미는 2024년 세계 매출의 38.69%를 창출했으며, 2024년 NICU에 대한 고급 보급과 조기 AI 도입을 배경으로 했습니다. 그럼에도 불구하고 FDA 일정 연장과 사이버 보안 규정 준수 비용은 당분간 성장을 억제합니다. 북미 태아 및 신생아 케어 장비 시장 규모는 2025년 37억 5,000만 달러에 이르고, CAGR 5.5%를 나타낼 전망이며, 2030년에는 49억 달러로 확대됩니다.
아시아태평양의 CAGR은 9.01%로 중국과 인도의 공중 보건에 대한 투자와 적극적인 기술 확대 프로그램에 의해 지원됩니다. Samsung Medison에 의한 Sonio의 9,300만 달러의 인수는 AI 태아 진단의 지배를 목표로 하는 이 지역의 야망을 나타내는 것입니다. 인도의 National Health Mission은 신생아 케어 패키지의 무료 배포를 계속하고 2급 도시에서의 장비 수요에 박차를 가하고 있습니다.
유럽은 헬륨 프리 MRI와 케이블리스 모니터를 지지하는 지속가능성 조달 규칙에 힘입어 한 자리대 중반의 견조한 확대를 유지합니다. 라틴아메리카는 비용을 최적화한 수입에 주력하고 중동은 관민 파트너십을 통해 투자하고 모체 태아 서비스를 향상시키고 있습니다.
기타 혜택:
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 상황
- 시장 개요
- 시장 성장 촉진요인
- 조산 및 저출생 체중아 출산 증가
- 저자원 환경에서의 POC 태아 모니터링 수요 증가
- AI를 활용한 예측 분석에 의한 신생아의 결과 개선
- NICU의 수용 능력 확대를 촉진하는 정부의 지원
- 감염 위험을 줄이기 위해 비접촉 광선 요법과 가온기로의 전환
- NICU 워크플로우에서 일회용 소모품 증가
- 시장 성장 억제요인
- 신규 의료장비의 승인에 관한 엄격한 규제 스케줄
- 고급 통합 NICW 워크스테이션에 필요한 고액의 초기 비용
- 일부 지역에서 훈련을 받은 신생아과 의사와 간호사가 부족
- 연결된 태아 모니터링 플랫폼의 사이버 보안 위험
- 가치/공급망 분석
- 규제 상황
- 기술 전망
- Porter's Five Forces 분석
- 공급기업의 협상력
- 구매자의 협상력
- 신규 참가업체의 위협
- 대체품의 위협
- 경쟁 기업간 경쟁 관계
제5장 시장 규모와 성장 예측
- 제품 유형별
- 태아 도플러
- 태아 자기 공명 영상(MRI) 장치
- 초음파 장치
- 태아 산소포화도 측정기
- 기타 태아 케어 장비
- 태아 자기 공명 영상(MRI) 장치
- 신생아 케어 장비
- 인큐베이터
- 신생아 모니터링 장치
- 광선 치료 장비
- 호흡 보조 및 모니터링 장치
- 기타 신생아 케어 장비
- 태아 도플러
- 최종 사용자별
- 병원
- 산과 클리닉 및 출산 센터
- 재택 케어 환경
- 모달리티별
- 독립형 디바이스
- 휴대용 및 핸드헬드 디바이스
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 시장 집중도
- 시장 점유율 분석
- 기업 프로파일
- Atom Medical Corp.
- Becton, Dickinson & Co.
- Dragerwerk AG & Co. KGaA
- GE HealthCare Technologies Inc.
- Koninklijke Philips NV
- Masimo Corp.
- Medtronic plc
- Natus Medical Inc.
- Phoenix Medical Systems Pvt Ltd
- Vyaire Medical Inc.
- Fisher & Paykel Healthcare Ltd
- ICU Medical
- Nihon Kohden Corp.
- Inspiration Healthcare Group plc
- Mennen Medical Group
- Fanem LTDA
- Bispectral Medical
- Heinen Lowenstein GmbH
- Utah Medical Products Inc.
제7장 시장 기회와 장래의 전망
JHS 25.11.17The fetal and neonatal care equipment market was valued at USD 9.68 billion in 2025 and is forecast to reach USD 13.15 billion by 2030, advancing at a 6.31% CAGR.
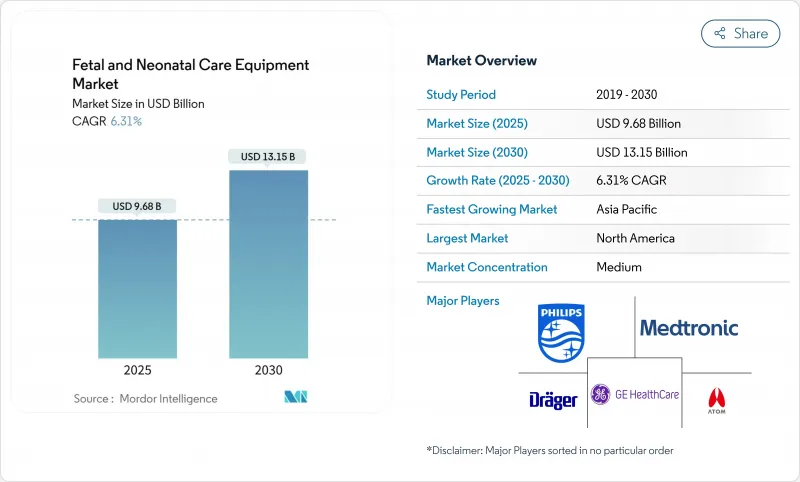
Growth stems from the convergence of higher preterm birth rates, wider use of AI-powered predictive analytics, and sizeable government investments that expand neonatal intensive-care capacity, especially in China where maternal mortality fell to 14.3 per 100,000 in 2024. Rising demand for wireless and portable monitors is dismantling long-standing reliance on tethered systems, while cybersecurity requirements influence product design and procurement decisions.Hospital buying power remains decisive, yet remote monitoring programs that enable early discharge are shifting revenue toward home-care channels. Competitive dynamics intensify as leading manufacturers integrate AI algorithms, secure-by-design architectures, and cloud connectivity, often through targeted acquisitions of start-ups that specialize in fetal diagnostics.
Global Fetal And Neonatal Care Equipment Market Trends and Insights
Increasing Number of Pre-Term and Low-Birth-Weight Deliveries
Preterm births rose to 10.4% of live births in the United States in 2023, a decade high that translates into sustained demand for advanced incubators, ventilators, and monitoring systems. The March of Dimes estimates annual economic losses of USD 25.2 billion from preterm births, sharpening hospital focus on early-intervention technologies. China's CARE-Preterm cohort noted a 10.74% mortality rate among very preterm infants, underscoring the clinical need for sophisticated support equipment. Higher maternal age intensifies risk: women aged 40 and older recorded a 14.8% preterm rate, creating a premium segment ready to pay for AI-enabled fetal diagnostics. These factors together lift demand for neonatal devices and recurring consumables that underpin the fetal and neonatal care equipment market.
Accelerating Demand for Point-of-Care Fetal Monitoring in Low-Resource Settings
Affordable innovations are redefining care pathways in emerging economies. A Malawian pilot showed the USD 56 Optoco external tocodynamometer produced clinically acceptable readings compared with premium systems. Uganda's deployment of the Moyo device improved intrapartum fetal heart-rate detection while maintaining user acceptance. NIH funding that rewards non-invasive prototypes incentivizes commercial scale-up of low-cost monitors nibib.nih.gov. China's neonatal mortality drop to 2.8 per mille in 2024 further illustrates how public funding lifts demand for accessible equipment. Collectively these trends enlarge the fetal and neonatal care equipment market by opening new buyer segments in low-resource settings.
Stringent Regulatory Timelines for Novel Device Approvals
FDA de-novo approvals fell to only 2 in 2025 versus 12 during 2024 as workforce reductions slowed reviews, adding months to commercialization schedules and deferring revenues. Harmonized Quality System Regulation amendments effective February 2026 introduce new documentation layers that raise compliance costs. Security documentation mandated under Section 524B further extends submission packages. Small innovators, often at the forefront of AI diagnostics, face resource constraints that can delay product launches and temper growth in the fetal and neonatal care equipment market.
Other drivers and restraints analyzed in the detailed report include:
- AI-Powered Predictive Analytics Improving Neonatal Outcomes
- Government Support to Boost NICU Capacity Expansion
- Cyber-Security Risks in Connected Fetal Monitoring Platforms
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Neonatal equipment controlled 56.23% of 2024 revenue in the fetal and neonatal care equipment market, anchored by incubators, ventilators, and thermoregulation systems sold into expanding NICU footprints. Incubators led unit shipments, with enhancements such as humidity-regulated micro-environments and integrated jaundice forecasting software like Draeger's BiliPredics elevating clinical value. Respiratory support devices capitalized on rising very-preterm survival rates; Vyaire's fabian Therapy evolution now offers closed-loop FiO2 control that reduces manual titration time. The fetal and neonatal care equipment market size for neonatal respiratory devices is projected to climb at 7.2% CAGR through 2030 as guidelines favor early non-invasive ventilation.
Fetal care devices, though smaller in absolute revenue, are on track for an 8.02% CAGR. AI-enhanced ultrasound, exemplified by GE's Vscan Air CL and Exo's Iris handheld with SweepAI algorithms, shortens exam times while improving detection fidelity. NIH funding that awarded USD 75,000 to six RADx finalists validates pipeline momentum in non-invasive pulse-oximetry and wearable cardiotocography solutions. The fetal and neonatal care equipment market size for pulse oximeters is forecast to expand 9.4% annually, reflecting demand for continuous, cable-free oxygen-saturation tracking during labor and the early neonatal period.
The Fetal and Neonatal Care Equipment Market Report is Segmented by Product Type (Fetal Care Equipment [Fetal Dopplers and More] and Neonatal Care Equipment [Incubators and More]), End User (Hospitals, Home-Care Settings, and More), Modality (Stand-Alone Devices and Portable/Handheld Devices), and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America generated 38.69% of global revenue in 2024 on the back of advanced NICU penetration and early AI adoption. Nevertheless, extended FDA timelines and cybersecurity compliance costs temper near-term growth. The fetal and neonatal care equipment market size in North America is set to rise from USD 3.75 billion in 2025 to USD 4.9 billion by 2030 at a 5.5% CAGR.
Asia Pacific charts a 9.01% CAGR, underpinned by Chinese and Indian public-health investments and aggressive technology scale-up programs. Samsung Medison's USD 93 million purchase of Sonio signals regional ambition to dominate AI-fetal diagnostics. India's National Health Mission continues to distribute free neonatal care packages that spur equipment demand in secondary-tier cities.
Europe maintains steady mid-single-digit expansion, helped by sustainability procurement rules that favor helium-free MRI and cableless monitors. Latin America focuses on cost-optimized imports, and the Middle East invests via public-private partnerships to upgrade maternal-fetal services.
- Atom Medical Corp.
- Beckton Dickinson
- Dragerwerk
- GE HealthCare Technologies Inc.
- Koninklijke Philips
- Masimo Corp.
- Medtronic
- Natus Medical
- Phoenix Medical Systems
- Vyaire Medical
- Fisher & Paykel Healthcare
- ICU Medical
- Nihon Kohden Corp.
- Inspiration Healthcare Group plc
- Mennen Medical Group
- Fanem LTDA
- Bispectral Medical
- Heinen + Lowenstein GmbH
- Utah Medical Products
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Number Of Pre-Term And Low-Birth-Weight Deliveries
- 4.2.2 Accelerating Demand For Point-Of-Care Fetal Monitoring In Low-Resource Settings
- 4.2.3 AI-Powered Predictive Analytics Improving Neonatal Outcomes
- 4.2.4 Government Support To Boost NICU Capacity Expansion
- 4.2.5 Shift Toward Non-Contact Phototherapy & Warmers To Reduce Infection Risk
- 4.2.6 Growth Of Single-Use Disposable Consumables In NICU Workflow
- 4.3 Market Restraints
- 4.3.1 Stringent Regulatory Timelines For Novel Device Approvals
- 4.3.2 High Upfront Cost Of Advanced Integrated NICU Workstations
- 4.3.3 Shortage Of Trained Neonatologists & Nurses In Certain Regions
- 4.3.4 Cyber-Security Risks In Connected Fetal Monitoring Platforms
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technology Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size and Growth Forecasts (Value-USD)
- By Product Type
- 5.1 Fetal Dopplers
- 5.1.1 Fetal Magnetic Resonance Imaging (MRI) Devices
- 5.1.1.1 Ultrasound Devices
- 5.1.1.2 Fetal Pulse Oximeters
- 5.1.1.3 Other Fetal Care Equipment
- 5.1.2 Neonatal Care Equipment
- 5.1.2.1 Incubators
- 5.1.2.2 Neonatal Monitoring Devices
- 5.1.2.3 Phototherapy Equipment
- 5.1.2.4 Respiratory Assistance & Monitoring Devices
- 5.1.2.5 Other Neonatal Care Equipment
- 5.1.1 Fetal Magnetic Resonance Imaging (MRI) Devices
- 5.1 Fetal Dopplers
- 5.2 By End-user
- 5.2.1 Hospitals
- 5.2.2 Maternity Clinics & Birthing Centers
- 5.2.3 Home-Care Settings
- 5.3 By Modality
- 5.3.1 Stand-alone Devices
- 5.3.2 Portable/Hand-held Devices
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.3.1 Atom Medical Corp.
- 6.3.2 Becton, Dickinson & Co.
- 6.3.3 Dragerwerk AG & Co. KGaA
- 6.3.4 GE HealthCare Technologies Inc.
- 6.3.5 Koninklijke Philips N.V.
- 6.3.6 Masimo Corp.
- 6.3.7 Medtronic plc
- 6.3.8 Natus Medical Inc.
- 6.3.9 Phoenix Medical Systems Pvt Ltd
- 6.3.10 Vyaire Medical Inc.
- 6.3.11 Fisher & Paykel Healthcare Ltd
- 6.3.12 ICU Medical
- 6.3.13 Nihon Kohden Corp.
- 6.3.14 Inspiration Healthcare Group plc
- 6.3.15 Mennen Medical Group
- 6.3.16 Fanem LTDA
- 6.3.17 Bispectral Medical
- 6.3.18 Heinen + Lowenstein GmbH
- 6.3.19 Utah Medical Products Inc.
7 Market Opportunities and Future Outlook
- 7.1 White-Space and Unmet-Need Assessment

















