
|
시장보고서
상품코드
1437948
주사제 시장 : 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Injectable Drugs - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
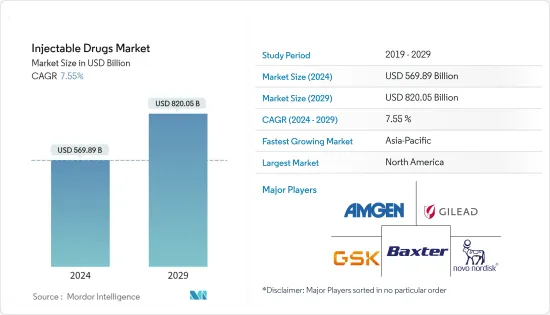
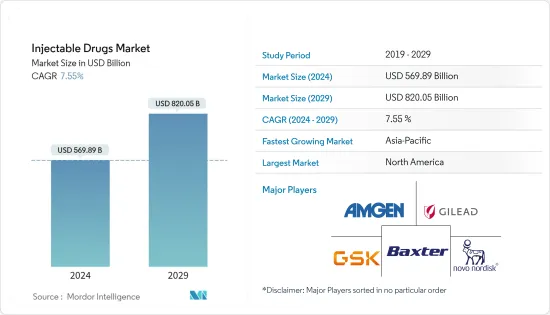
주사제(Injectable Drugs) 시장 규모는 2024년에 5,698억 9,000만 달러로 추정되며, 2029년까지 8,200억 5,000만 달러에 이를 것으로 예측되며, 예측 기간(2024-2029년) 동안 7.55%의 CAGR로 추이하며 성장할 전망입니다.
COVID-19는 연구 대상 시장 성장에 영향을 미쳤습니다. 팬데믹 기간 동안 기업들은 COVID-19 백신을 개발하기 위해 약물을 개발하고 협력과 승인을 모색했습니다. 이러한 백신은 주로 주사제 형태로 제공되어 시장 성장을 주도했습니다. 예를 들어, 2020년 5월 아스트라제네카는 미국 보건부의 생물의학첨단연구개발국(BARDA)으로부터 옥스퍼드 대학의 COVID-19 백신 개발, 생산 및 공급을 위해 10억 달러 이상의 자금을 지원받았습니다. 또한 COVID-19 제한 조치가 해제되고 기업 활동이 재개됨에 따라 시장은 예측 기간 동안 성장할 것으로 예상됩니다.
생명공학 기술로 설계된 항암제 개발에 대한 R&D 집중, 생물학적 제제용 프리필드 주사기 사용의 급속한 증가, 주사제 공급을 증가시킬 것으로 예상되는 가치 사슬 전반의 아웃소싱 활동 증가 등의 요인이 시장 성장에 힘을 보태고 있습니다.
암, 당뇨병, 심혈관 질환 등과 같은 만성 질환의 유병률 증가는 시장 성장을 이끄는 주요 요인입니다. 예를 들어, 2022년 5월 BioMed Central Journal에 발표된 기사에 따르면 인도에서 암을 앓고 있는 사람은 2,670만 명이며 2025년에는 이 숫자가 2,980만 명으로 증가할 것으로 예상됩니다. 또한 IDF가 발표한 2022년 통계에 따르면 프랑스에서는 약 390만 명이 당뇨병을 앓고 있으며 이 수치는 2030년에는 410만 명, 2045년에는 420만 명에 이를 것으로 예상됩니다. 따라서 인구의 만성 질환에 대한 높은 부담은 효과적이고 안전한 주사제에 대한 수요를 증가시켜 시장 성장을 촉진 할 것으로 예상됩니다.
또한 2021년 12월 영국 보건안보국이 발표한 보고서에 따르면 2020년 영국에서는 약 97,740명이 HIV에 감염된 것으로 추정되며, 2020년에는 4,660명이 자신의 감염 사실을 인지하지 못하고 있어 예측 기간 동안 시장 성장을 촉진할 것으로 예상되는 주사 가능한 HIV 약물 및 제제에 대한 수요가 증가할 것으로 전망됩니다. 또한 2021년 8월 국제 건강 형평성 저널에 발표된 논문에 따르면 60세 이상 노인 인구의 75.8%가 심장병, 암, 만성 폐질환 등 하나 이상의 만성 질환으로 고생하고 있는 것으로 나타났습니다.
또한, 새로운 치료 클래스의 주사제 개발에 대한 기업들의 관심이 높아지면서 의사와 환자들의 채택과 시장에서의 가용성이 증가할 것으로 예상되며, 이는 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, 2022년 9월 미국 식품의약국(FDA)은 바이오로직스 허가 신청(BLA)을 승인하고 알파 만노시증 치료를 위한 효소 대체 요법인 벨마나제 알파를 우선 검토 대상으로 지정했습니다. 또한 2021년 12월, 미국 식품의약국(FDA)은 성적으로 획득한 HIV의 위험을 줄이기 위한 노출 전 예방을 위해 체중 35kg 이상의 청소년을 대상으로 아프레튜드(성분명: 카보테그라비르 서방형 주사 현탁액)를 승인했습니다.
또한, 생명공학 항암제 개발이 활발해지면서 시장 성장도 가속화되고 있습니다. 예를 들어, 2022년 4월 중국 국가약품감독관리국(NMPA)은 식도암의 일종인 국소 진행성 또는 전이성 식도 편평세포암(ESCC) 환자 치료를 위해 베이진의 티셀리주맙을 승인했습니다. 또한 2021년 12월 중국 국가약품감독관리국(NMPA)의 의약품평가센터(CDE)는 비소세포폐암(NSCLC), 요로상피암(UC) 및 기타 일부 암을 포함한 고형 종양 환자를 대상으로 에노블리투주맙(TJ271)과 펨브롤리주맙(Keytruda)을 병용하는 중국 내 2상 시험 개시를 위한 I-Mab의 IND 제출을 승인했습니다.
그러나 재고 관리와 관련된 비용이 많이 드는 비용과 대체 약물 전달 메탄의 가용성은 예측 기간 동안 시장 성장을 방해할 수 있습니다.
주사제 시장 동향
종양학은 예측 기간 동안 상당한 시장 점유율을 획득할 것으로 예상
종양학 부문은 인구의 암 유병률 증가 및 생명 공학 방법을 사용한 첨단 암 치료제 개발과 같은 요인으로 인해 예측 기간 동안 주사제 시장에서 상당한 성장을 보일 것으로 예상됩니다. 예를 들어, 글로보칸 2020에 따르면 중국은 2020년에 4,568,754건의 새로운 암 사례가 보고되었고, 5년간 9,294,006건의 암 사례가 유행했습니다. 또한 같은 보고서에 따르면 2030년에는 5,811,629건, 2040년에는 6,845,787건의 암 사례가 발생할 것으로 예상했습니다. 마찬가지로 같은 출처에 따르면 일본은 2020년에 1,028,658건의 새로운 암 사례가 보고되었으며, 5년간 총 유병 암 사례 수는 2,710,728건입니다. 같은 보고서는 2030년에는 암 발병 건수가 1,110,549건, 2040년에는 1,128,057건에 달할 것으로 예상했습니다. 따라서 인구의 암 발병 건수 증가는 주사형 암 치료제에 대한 수요를 촉진하여 시장 성장을 촉진할 것으로 예상됩니다.
또한 기업 활동의 증가와 제품 출시 증가도 이 부문의 성장에 기여하고 있습니다. 예를 들어, 2022년 11월 미국 식품의약국은 전이성 비소세포폐암(NSCLC) 성인 환자를 위해 트레멜리무맙과 더발루맙 및 백금 기반 화학요법 병용요법을 승인했습니다. 또한, 2022년 5월에는 미국에서 다발성 골수종 및 맨틀세포 림프종과 같은 특정 유형의 암 치료에 사용되는 보르테조밉 주사를 출시했습니다.
따라서 암 부담 증가와 신제품 출시가 예측 기간 동안 시장 성장을 가속할 것으로 예상됩니다.
북미는 주사제 시장에서 가장 큰 시장 점유율을 유지
북미는 암, 당뇨병, 심혈관 질환 등 만성 질환의 이환율이 높고, 견고한 헬스케어 인프라, 이 지역의 주요 기업에 의해 예측 기간에 걸쳐 주사제 시장을 독점할 것으로 예상되고 있습니다.
게다가 신제품 출시 횟수 증가, 연구개발에 대한 대규모 투자, 다양한 유형의 암 치료를 위한 병원 주사제 채택 증가도 이 지역 시장 성장에 기여하고 있습니다.
미국 암 협회의 2022년 보고서에 따르면 2022년 미국에서 190만 건 이상의 새로운 암 사례가 발생할 것으로 예상됩니다. 또한 같은 자료에 따르면 암 위험은 나이가 들수록 급격히 증가합니다. 미국에서는 암 환자의 80%가 55세 이상이며, 57%는 65세 이상입니다. 따라서 노인 인구의 증가는 연구 대상 시장에 큰 영향을 미칠 것으로 예상됩니다. 또한 2021년 12월 국제 당뇨병 연맹의 보고서에 따르면 미국에서는 약 3,220만 명이 당뇨병을 앓고 있는 것으로 나타났습니다. 이 수치는 2045년까지 3,630만 명으로 증가할 것으로 예상됩니다. 이러한 당뇨병 환자 수의 증가는 주사제에 대한 수요를 증가시켜 시장 성장을 촉진할 것으로 예상됩니다.
만성 질환에 대한 효과적인 치료제에 대한 수요가 증가함에 따라 제품 출시, 인수 합병, 파트너십 및 전략적 협업과 같은 회사 활동이 국내에서 연구 된 시장 성장을 촉진 할 것으로 예상됩니다. 예를 들어, 로슈는 2022년 5월 유방암 치료를 위해 퍼제타(퍼투주맙)와 허셉틴(트라스투주맙)을 히알루로니다아제와 결합한 페스고(PHESGO)를 출시했습니다. 또한 2022년 2월 미국 식품의약국(FDA)은 12세 이상 성인 및 소아 환자의 유전성 혈관부종(HAE) 발작 예방을 위해 다케다의 타크자이로(라나델루맙-플라이오) 1회용 프리필드 시린지(PFS) 주사를 승인했습니다.
따라서, 상기 요인들에 의해 주사제 시장은 예측 기간 동안 이 지역에서 성장할 것으로 예상됩니다.
주사제 산업 개요
주사제 시장의 경쟁은 중간 정도입니다. 주요 시장 플레이어는 GlaxoSmithKline PLC, Baxter International Inc., Amgen Inc.를 포함합니다. 시장의 주요 기업 중 일부는 제조 및 유통을 용이하게 하는 특정 제품에 대해 통합 파트너십을 맺고 있는 기업도 있습니다. 다양한 기업들이 생산 능력을 확대하고 확대하는 것도 시장 전체를 밀어 올리는 데 도움이 됩니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 서포트
목차
제1장 서론
- 조사 전제 조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진 요인
- 바이오 테크놀로지 공학에 의한 항암제의 개발을 향한 연구 개발의 관심 확대
- 생물 제제용 프리필드 주사기 사용이 급속히 확대
- 가치사슬 전체에 걸친 아웃소싱 활동 증가에 의한 주사용 제품공급 증가
- 시장 성장 억제 요인
- 재고 관리에 따른 고액의 비용
- 대체 약물전달 방법의 이용 가능성
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체 제품의 위협
- 기업 간 경쟁 정도
제5장 시장 세분화
- 분자유형별
- 저분자
- 고분자
- 약제 클래스별
- 혈액 인자
- 사이토카인
- 펩티드 호르몬
- 면역글로불린
- 단클론항체(mAb)
- 인슐린
- 다른 약물 클래스
- 용도별
- 종양학
- 신경내과
- 심혈관 질환
- 자가면역질환
- 감염증
- 통증
- 기타 용도
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아 태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아 태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 개요
- Novo Nordisk AS
- Amgen Inc.
- Baxter International Inc.
- Gilead Sciences Inc.
- GlaxoSmithKline PLC
- Johnson &Johnson
- Merck &Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi SA
- AbbVie
- F. Hoffmann-La Roche Ltd
제7장 시장 기회 및 향후 동향
LYJThe Injectable Drugs Market size is estimated at USD 569.89 billion in 2024, and is expected to reach USD 820.05 billion by 2029, growing at a CAGR of 7.55% during the forecast period (2024-2029).
COVID-19 impacted the growth of the market studied. During the pandemic, companies were developing drugs and seeking collaborations and approvals to develop a vaccine for COVID-19. These vaccines were majorly given in the injectable form, driving the market growth. For instance, in May 2020, AstraZeneca received more than USD 1 billion in funding from the US Health Department's Biomedical Advanced Research and Development Authority (BARDA) for the development, production, and delivery of the University of Oxford's COVID-19 vaccine. Moreover, with the released COVID-19 restrictions and resumed company activities, the market is expected to grow over the forecast period.
The factors such as rising R&D focus on developing biotechnology-engineered anti-cancer drugs, rapid growth in the usage of pre-filled syringes for biologics, and increased outsourcing activities across the value chains expected to raise the supply of injectable drugs are boosting the market growth.
The rising prevalence of chronic diseases such as cancer, diabetes, cardiovascular diseases, and others is the key factor driving the market growth. For instance, from an article published in BioMed Central Journal in May 2022, it has been observed that 26.7 million people in India were suffering from cancer and this number is projected to increase to 29.8 million by 2025. Also, according to 2022 statistics published by the IDF, about 3.9 million people were living with diabetes in France, and this number is projected to reach 4.1 million by 2030 and 4.2 million by 2045. Thus, the high burden of chronic diseases among the population is anticipated to increase the demand for effective and safe injectable drugs hence propelling the market growth.
Additionally, a report published by the UK Health Security Agency in December 2021, stated that in 2020, an estimated 97,740 people were living with HIV in England and an estimated 4,660 in 2020 were unaware of their infection, indicating the demand for injectable HIV drugs and formulations, which are anticipated to augment the market growth over the forecast period. Also, from an article published in the International Journal for Equity in Health in August 2021, it was observed that 75.8% of the elderly population aged 60 years and above were troubled by one or more chronic diseases such as heart disease, cancer, chronic lung diseases, and others.
Furthermore, the rising focus of companies on developing injectable drugs for new therapeutic classes is expected to increase their adoption by physicians and patients and their availability in the market, which in turn is anticipated to fuel market growth. For instance, in September 2022, the USFDA accepted the Biologics License Application (BLA) and granted Priority Review designation to Chiesi Global Rare Diseases, velmanase alfa, an enzyme replacement therapy, for the treatment of alpha-mannosidosis. Also, in December 2021, the USFDA approved Apretude (Cabotegravir extended-release injectable suspension) for adolescents weighing at least 35 kg for pre-exposure prophylaxis to reduce the risk of sexually acquired HIV.
Moreover, the rising development of bio-engineered anti-cancer drugs is also increasing the market growth. For instance, in April 2022, the China National Medical Products Administration (NMPA) approved BeiGene's tislelizumab to treat patients with locally advanced or metastatic oesophageal squamous cell carcinoma (ESCC), a type of oesophageal cancer. Also, in December 2021, the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) approved I-Mab's IND submission for the initiation of a phase 2 trial in China for enoblituzumab (also known as TJ271) in combination with pembrolizumab (Keytruda) in patients with solid tumors, including non-small cell lung cancer (NSCLC), urothelial carcinoma (UC), and other selected cancers.
However, the high expenses associated with inventory management and the availability of alternate drug delivery methanes are likely to hinder the market growth over the forecast period.
Injectable Drugs Market Trends
Oncology Is Expected to Have Significant Market Share During the Forecast Period
The oncology segment is expected to witness significant growth in the injectable drugs market over the forecast period, owing to factors such as the rising prevalence of cancer among the population and the development of advanced cancer drugs using bio-engineered methods. For instance, according to Globocan 2020, China reported 4,568,754 new cancer cases in 2020, and 9,294,006 cancer cases were prevalent for five years. Further, the same report projected that cancer cases will reach 5,811,629 by 2030 and 6,845,787 by 2040. Similarly, as per the same source, Japan reported 1,028,658 new cancer cases in 2020, and the total number of five-year prevalent cancer cases was 2,710,728. The same report projected the number of cancer cases to reach 1,110,549 by 2030 and 1,128,057 by 2040. Thus, the expected rise in the number of cancer cases among the population is projected to propel the demand for injectable cancer drugs, which, in turn, is anticipated to fuel market growth.
Furthermore, the rise in company activities and increasing product launches are also contributing to this segment's growth. For instance, in November 2022, the Food and Drug Administration approved tremelimumab in combination with durvalumab and platinum-based chemotherapy for adult patients with metastatic non-small cell lung cancer (NSCLC). Also, in May 2022, Gland Pharma launched Bortezomib injection, which is used to treat certain types of cancer, such as multiple myeloma and mantle cell lymphoma, in the United States.
Thus, the increasing burden of cancer and the launch of novel products are expected to drive the market's growth over the forecast period.
North America Holds the Largest Market Share of the Injectable Drugs Market
North America is expected to dominate the injectable drugs market over the forecast period owing to the high prevalence of chronic diseases such as cancer, diabetes, and cardiovascular diseases, robust healthcare infrastructure, and major players in the region.
In addition, the increasing number of novel product launches, huge investments in R&D, and the increased adoption of injectable drugs in hospitals to treat different types of cancer are also contributing to the market growth in the region.
According to the American Cancer Society's 2022 report, over 1.9 million new cancer cases are expected to be recorded in the United States in 2022. Also, per the same source, cancer risk rises dramatically as one ages. In the United States, 80% of cancer patients are 55 years or older, with 57% being 65 years or older. Thus, the growing geriatric population is expected to impact the market studied significantly. Further, according to the International Diabetes Federation's report of December 2021, about 32.2 million people were living with diabetes in the United States. This number is estimated to increase to 36.3 million by 2045. This increase in the diabetes patient pool is expected to drive demand for injectable drugs, which will boost growth in the market.
With the growing demand for effective therapeutics for chronic diseases, company activities such as product launches, mergers and acquisitions, partnerships, and strategic collaborations are expected to augment growth in the market studied in the country. For instance, in May 2022, Roche launched PHESGO, a combination of Perjeta (pertuzumab) and Herceptin (trastuzumab) with hyaluronidase to treat breast cancer. Also, in February 2022, the United States Food and Drug Administration (FDA) approved Takeda's TAKHZYRO (lanadelumab-flyo) injection single-dose prefilled syringe (PFS) to prevent attacks of hereditary angioedema (HAE) in adult and pediatric patients 12 years of age and older.
Therefore, due to the factors mentioned above, the injectable drugs market is expected to grow in the region over the forecast period.
Injectable Drugs Industry Overview
The injectables drugs market is moderately competitive. The key market players include GlaxoSmithKline PLC, Baxter International Inc., and Amgen Inc. Some of the major players in the market have consolidated partnerships for certain products that allow for ease of manufacturing and distribution. The expansion of different companies to increase their production capacities also helps boost the overall market.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising R&D Focus on the Development of Biotechnology-engineered Anti-cancer Drugs
- 4.2.2 Rapid Growth in the Usage of Pre-filled Syringes for Biologics
- 4.2.3 Increased Outsourcing Activities Across Value Chain Expected to Boost Supply of Injectable Products
- 4.3 Market Restraints
- 4.3.1 High Expenses Associated with Inventory Management
- 4.3.2 Availability of Alternate Drug Delivery Methods
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Molecule Type
- 5.1.1 Small Molecule
- 5.1.2 Large Molecule
- 5.2 By Drug Class
- 5.2.1 Blood Factors
- 5.2.2 Cytokines
- 5.2.3 Peptide Hormone
- 5.2.4 Immunoglobulin
- 5.2.5 Monoclonal Antibodies (mAbs)
- 5.2.6 Insulin
- 5.2.7 Other Drug Classes
- 5.3 By Application
- 5.3.1 Oncology
- 5.3.2 Neurology
- 5.3.3 Cardiovascular Diseases
- 5.3.4 Autoimmune Diseases
- 5.3.5 Infectious Diseases
- 5.3.6 Pain
- 5.3.7 Other Applications
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Novo Nordisk AS
- 6.1.2 Amgen Inc.
- 6.1.3 Baxter International Inc.
- 6.1.4 Gilead Sciences Inc.
- 6.1.5 GlaxoSmithKline PLC
- 6.1.6 Johnson & Johnson
- 6.1.7 Merck & Co. Inc.
- 6.1.8 Novartis AG
- 6.1.9 Pfizer Inc.
- 6.1.10 Sanofi SA
- 6.1.11 AbbVie
- 6.1.12 F. Hoffmann-La Roche Ltd

















