
|
시장보고서
상품코드
1435211
디지털 PCR : 세계 시장 점유율 분석, 산업 동향 및 통계, 성장 동향 예측(2024-2029년)Global Digital PCR - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
세계의 디지털 PCR 시장 규모는 2024년에 6억 9,000만 달러로 추정되고, 2029년까지 15억 1,000만 달러에 이를 것으로 예측되며, 예측 기간(2024-2029년) 동안 16.74%의 연평균 복합 성장률(CAGR)로 성장할 전망입니다.

신형 코로나바이러스 감염(COVID-19)의 진단에 가장 흔히 사용되고 신뢰할 수 있는 검사는 비인두 봉제액 및 기타 상부기도 검체(목의 봉제액, 최근에는 면역검정용 타액 등)를 사용하여 수행되는 드롭렛 디지털 PCR 검사입니다. 따라서 디지털 PCR을 이용한 질병 진단은 팬데믹 관리의 주요 초점으로 부상했습니다. 2021년 10월, 호흡기 샘플에서 SARS CoV-2 단백질을 검출하는 COVID-19증 항원 검사가 긴급 사용 허가를 받았습니다. 따라서 디지털 드롭렛 PCR 수요가 크게 증가했습니다. 이들은 더 민감하고 질병을 신속하게 진단합니다. 또한 2020년 4월 바이오 래드는 Droplet Digital PCR SARS-CoV-2 Test Kit의 FDA 긴급 사용 허가를 받았습니다. 따라서 디지털 PCR 수요는 시장 성장을 가속할 것으로 예상됩니다.
시장 성장에 기여하는 주요 요인은 암이나 감염과 같은 질병의 진단 및 스크리닝 검사 증가, 부모의 검사 및 유전자 연구 수요 증가입니다. GLOBOCAN의 보고서에 따르면, 2020년에는 세계에서 약 1,929만명이 암을 앓고 있습니다. 그 수는 2040년까지 2,889만 명으로 증가할 것으로 추정됩니다. 국제 당뇨병 연맹의 당뇨병 아틀라스 10판 2021에 따르면, 2021년에는 세계 약 5억 3,700만 명의 성인이 당뇨병임이 밝혀졌습니다. 그 수는 2030년까지 6억 4,300만 명, 2045년까지 7억 8,300만 명으로 증가할 것으로 예측됩니다. 이러한 질병의 발생률이 증가함에 따라 디지털 PCR의 사용이 증가할 것으로 예상됩니다.
따라서, 상기 요인은 예측 기간 동안 시장 성장을 가속할 것으로 예상됩니다. 그러나 디지털 PCR 장치의 비용이 높기 때문에 시장 성장이 억제될 것으로 예상됩니다.
디지털 PCR 시장 동향
드롭렛 디지털 PCR 부문이 세계 시장을 독점
드롭렛 디지털 PCR(ddPCR)은 물-오일 에멀젼 액적 기술을 기반으로 디지털 PCR을 수행하는 방법입니다. 샘플은 20,000개의 액적으로 분획되고, 각 액적에서 주형 분자의 PCR 증폭이 수행됩니다. ddPCR 기술은 대부분의 표준 TaqMan 프로브 기반 분석에 사용되는 것과 유사한 시약과 워크플로우를 사용합니다. 대규모 샘플의 분할은 ddPCR 기술의 중요한 측면입니다. 위에서 언급한 ddPCR의 장점과는 별도로, 이 분야의 성장을 추진하는 요인으로는 암과 감염증의 이환율 증가, 자금 조달 증가 및 생명공학 분야의 진보를 들 수 있습니다.
액적 디지털 PCR의 성장은 주로 기술의 발전과 수요 증가로 인한 것입니다. 예를 들어 BioRAD 제품 QX200 드롭렛 디지털 PCR 시스템은 디지털 형식으로 표적 DNA의 절대 정량을 제공합니다. 이들 제품은 유전자 발현 수준을 절묘한 정확도로 측정하고, 희귀한 DNA 표적 복사를 검출하며, 비교할 수 없는 정확도로 복사 수의 변동을 결정합니다. 2020년 국립위생 연구소에 게재된 기사에 따르면, 현재 COVID-19 감염증의 유행중에, 비말 디지털 PCR은 신형 COVID-19 감염증 진단의 골드 스탠다드로 간주되고 있어 그 결과, 비말 디지털 PCR에 대한 시장 수요가 발생하고 있으며, 시장의 성장을 가속합니다.
또한, 기업은 액적 기술과 관련된 제품을 출시하고 있습니다. 2020년 5월 Bio-Rad Laboratories Inc.는 액적 디지털 PCR SARS-CoV-2 검사 키트에 대해 미국 식품의약국(FDA)의 긴급 사용 허가를 받았습니다. 또한 2019년 바이오 래드는 QX ONE 액적 디지털 PCR 시스템을 출시했습니다.
그러므로 위의 요인은 예측 기간 동안 조사 대상 부문의 성장을 가속할 것으로 예상
북미가 시장을 독점하고 있으며 예측기간 동안도 마찬가지로 추이할 것으로 예상
북미는 예측 기간 동안 전체 시장을 지배할 것으로 예상됩니다. 시장의 성장은 주요 기업의 존재, 지역의 만성 질환의 높은 유병률, 확립된 건강 관리 인프라 등의 요인 때문입니다. 또한, 유익한 정부의 이니셔티브 및 조사 파트너십의 수가 증가하는 것은 시장 성장을 가속할 것으로 예상되는 추진력의 일부입니다. 이 지역에서는 지원 헬스케어 정책, 수많은 환자 수, 개발된 헬스케어 시장으로 미국이 가장 큰 점유율을 차지하고 있습니다.
미국암협회의 2021년 보고서에 따르면 2021년에 약 1,898,160건의 암이 진단되었으며, 그 중에는 미국에서 남성 970,250명, 여성 927,910명이 포함되어 있으며, 미국인 608,570명이 에 의해 사망했습니다. 따라서 암의 이환율이 높기 때문에 이 나라에서는 진단과 치료 수요도 높아 조사 대상 미국 시장의 성장을 견인할 것으로 예상되고 있습니다.
또한, 이러한 성장은 정교한 진단 실험실의 존재와 새로운 분석 기술의 높은 수용률에 기인한다고 생각됩니다. 미국의 학술계 및 생명공학 산업에서 유전공학 및 게놈 조사의 추세가 증가하고 있습니다. 또한, 게놈 조사 증가는 주로 만성 질환 관리를 위한 표적화된 정밀의료에 대한 높은 수요와 질병의 유전적 및 분자적 기초를 이해할 필요성에 의해 촉진되고 있습니다.
이러한 노력은 제약 기업 및 생명 공학 기업에게 유전자 기반 진단 및 치료법 개발 및 판매에 유리한 환경을 제공합니다. 이는 디지털 PCR 기술의 성장을 가속할 것으로 예상됩니다. 디지털 PCR 기술은 창약, 독성, 효능 연구뿐만 아니라 일반적인 생명 과학 연구에도 광범위하게 응용되고 있기 때문입니다.
디지털 PCR 산업 개요
디지털 PCR 시장은 적당한 경쟁이 있고 몇몇 주요 기업으로 구성됩니다. 현재 시장을 독점하는 기업으로는 Bio-Rad Laboratories Inc., Thermo Fisher Scientific, Sysmex Corporation, Fluidigm Corporation, JN Medsys, QIAGEN, Merck KGaA, Stilla, Avance Biosciences 등이 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제 조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 감염증 및 암의 유병률의 상승
- 바이오 테크놀러지 분야에서의 기술의 진보
- 시장 성장 억제요인
- 디지털 PCR 디바이스의 고비용
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자 및 소비자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계의 강도
제5장 시장 세분화
- 제품 유형별
- 디지털 PCR 기기
- 소모품 및 시약
- 소프트웨어 및 서비스
- 기술별
- 액적 디지털 PCR
- 비밍 디지털 PCR
- 용도별
- 임상 진단
- 법의학
- 연구
- 기타 용도
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Bio-Rad Laboratories Inc.
- Thermo Fisher Scientific Inc
- Sysmex Corporation
- Fluidigm Corporation
- JN Medsys
- QIAGEN
- Merck KGaA
- Avance Biosciences
- Takara Bio Inc.
- Meridian Bioscience
제7장 시장 기회 및 앞으로의 동향
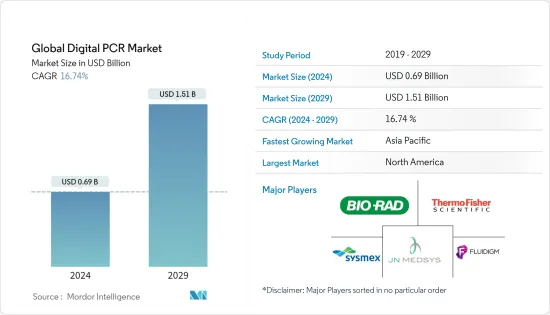
AJY 24.03.05The Global Digital PCR Market size is estimated at USD 0.69 billion in 2024, and is expected to reach USD 1.51 billion by 2029, growing at a CAGR of 16.74% during the forecast period (2024-2029).
The most commonly used and reliable test for diagnosis of COVID-19 has been the Droplet Digital PCR test performed using nasopharyngeal swabs or other upper respiratory tract specimens, including throat swabs or, more recently, saliva for immunology assays. Thus, disease diagnosis using digital PCR emerged as a major focus for the management of the pandemic. In October 2021, the COVID-19 antigen test that detects SARS CoV-2 proteins in respiratory samples received an emergency-use authorization. Thus, the demand for digital droplet PCR increased significantly. These are more sensitive, and rapid in diagnosing the disease. In addition, in April 2020, Bio-Rad received FDA emergency use authorization for the Droplet Digital PCR SARS-CoV-2 Test Kit. Thus the demand for the Digital PCR is expected to drive the growth of the market.
The major factor attributing to the growth of the market is the rising diagnostics and screening tests of diseases like cancer, and infectious diseases, and the growing demand for parental testing and gene study. According to the GLOBOCAN report, in 2020, around 19.29 million people in the world are affected by cancer. The number is estimated to rise to 28.89 million cases by 2040. According to the International Diabetes Federation Diabetes Atlas Tenth edition 2021, in 2021, around 537 million adults all over the world were found to have diabetes; with the numbers projected to grow to 643 million by 2030 and 783 million by 2045. With the rising incidence of such diseases, the usage of the Digital PCR is expected to grow.
Thus the above mentioned factors are expected to drive the growth of the market during the forecast period. However, high cost of digital PCR devices are expected to restrain the growth of the market.
Digital PCR Market Trends
Droplet Digital PCR Segment to Dominate the Market Globally
Droplet digital PCR (ddPCR) is a method for performing digital PCR that is based on water-oil emulsion droplet technology. A sample is fractionated into 20,000 droplets, and PCR amplification of the template molecules occurs in each droplet. The ddPCR technology uses reagents and workflows similar to those used for most standard TaqMan probe-based assays. The massive sample partitioning is a key aspect of the ddPCR technique. Apart from the above-mentioned advantages of the ddPCR, factors that are propelling the segment growth are the increasing prevalence of cancer, and infectious diseases, and increasing funding and advancements in the biotechnology sector.
The growth of droplet digital PCR is mainly attributed to technological advancement and increasing demand. For instance, BioRAD'sproduct QX200 Droplet Digital PCR System provides absolute quantification of target DNA in digital form. These product measures gene expression level with exquisite precision detects rare DNA target copies and determines copy number variation with unrivaled accuracy. According to an article published in the National Institute of Health 2020, currently, during the COVID-19 pandemic droplet digital PCR is considered to be a gold standard for the diagnosis of COVID-19 infections, thus triggering the market demand for droplet digital PCR, boosting market growth.
Moreover, companies are launching products associated with droplet technology. In May 2020, Bio-Rad Laboratories Inc. received the United States Food and Drug Administration (FDA) Emergency Use Authorization for Droplet Digital PCR SARS-CoV-2 Test Kit. In addition, in 2019, Bio-Rad launched QX ONE droplet digital PCR system.
Hence, the above-mentioned factors are expected to boost the growth of the studied segment during the forecast period
North America Dominates the Market and Expected to do Same over the Forecast Period
North America is expected to dominate the overall market throughout the forecast period. The market growth is due to the factors such as the presence of key players, the high prevalence of chronic diseases in the region, and the established healthcare infrastructure. Furthermore, beneficial government initiatives and an increase in the number of research partnerships are some of the drivers expected to increase market growth. In this region, the United States has the maximum share due to supportive healthcare policies, high number of patients, and a developed healthcare market.
According to the American Cancer Society's 2021 report, about 1,898,160 cancer cases were diagnosed in 2021, which included 970,250 males and 927,910 females in the United States and 608,570 Americans who died from it. Thus, due to the high prevalence of cancer, the demand for diagnostics and treatment is also high in the country which is expected to drive the growth in the studied market in the United States.
Moreover, the growth can be attributed to the presence of sophisticated diagnostic laboratories and the high acceptance rate of novel assay technologies. There is a growing trend of genetic engineering and genomic research in academia and biotechnology industries of the United States. Moreover, increasing genomic research is primarily fueled by the high demand for targeted and precision medicine for the management of chronic disorders and the need for understanding the genetic and molecular basis of disease.
Initiatives like these are presenting a favorable environment for pharmaceutical and biotechnology firms to develop and market gene-based diagnostics and therapeutics. This, in turn, is projected to fuel the growth of digital PCR technologies, due to their wide applications in drug discovery, toxicity, and efficacy studies, as well as in general life science research.
Digital PCR Industry Overview
The digital PCR market is moderately competitive and consists of several major players. Some of the companies which are currently dominating the market are Bio-Rad Laboratories Inc., Thermo Fisher Scientific, Sysmex Corporation, Fluidigm Corporation, JN Medsys, QIAGEN, Merck KGaA, Stilla, and Avance Biosciences.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rise in Prevalence of Infectious Diseases and Cancer
- 4.2.2 Technological Advancements in the Field of Biotechnology
- 4.3 Market Restraints
- 4.3.1 High Cost of Digital PCR Devices
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Product Type
- 5.1.1 Digital PCR Equipment
- 5.1.2 Consumables and Reagents
- 5.1.3 Software and Services
- 5.2 By Technology
- 5.2.1 Droplet Digital PCR
- 5.2.2 BEAMing Digital PCR
- 5.3 By Application
- 5.3.1 Clinical Diagnostics
- 5.3.2 Forensics
- 5.3.3 Research
- 5.3.4 Other Applications
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle-East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle-East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Bio-Rad Laboratories Inc.
- 6.1.2 Thermo Fisher Scientific Inc
- 6.1.3 Sysmex Corporation
- 6.1.4 Fluidigm Corporation
- 6.1.5 JN Medsys
- 6.1.6 QIAGEN
- 6.1.7 Merck KGaA
- 6.1.8 Avance Biosciences
- 6.1.9 Takara Bio Inc.
- 6.1.10 Meridian Bioscience

















