
|
시장보고서
상품코드
1438095
피부암 진단 및 치료 시장 : 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Global Skin Cancer Diagnostics and Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
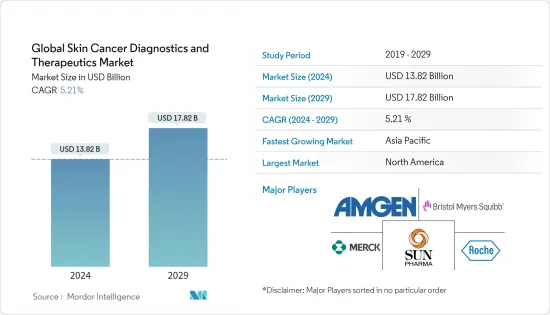
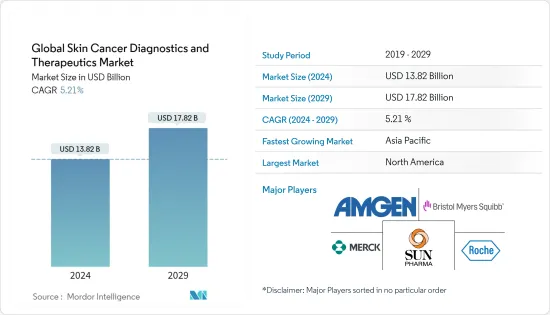
세계의 피부암 진단 및 치료(Skin Cancer Diagnostics and Therapeutics) 시장 규모는 2024년에 138억 2,000만 달러로 추정되며, 2029년까지 178억 2,000만 달러에 달할 것으로 예측되고 있으며, 예측 기간(2024-2029년) 동안 5.21%의 CAGR로 추이하며 성장할 전망입니다.
COVID-19 팬데믹의 발발은 연구 대상 시장에 큰 영향을 미쳤습니다. 팬데믹이 시작된 후 세계보건기구(WHO)의 지침에 따라 만성 질환 환자는 실내에 머물도록 권고했습니다. 따라서 이는 피부암 진단 시장을 방해했습니다. 하지만 COVID-19로 인한 치료 지연이 비흑색종 피부암(NMSC) 또는 흑색종 암 환자에게 미치는 영향을 연구하는 연구가 급증했습니다. 2021년 8월에 발표된 'COVID-19 팬데믹이 피부암 환자의 삶의 질에 미치는 영향' 연구에서는 면역 저하/억제 상태와 기저 종양 질환 및 부담으로 인해 암 환자는 중증 COVID-19 질환으로 발전하여 중환자실에서 치료가 필요할 위험이 높아질 수 있다고 언급했습니다. 따라서 팬데믹 기간 동안 피부암 발병률이 증가하면서 이에 대한 진단이 증가하고 치료를 위한 새로운 첨단 치료제에 대한 수요가 창출되었습니다. 따라서 팬데믹은 팬데믹 기간 동안 피부암 진단 및 치료제에 긍정적인 영향을 미칠 것으로 예상됩니다.
시장 성장을 이끄는 특정 요인으로는 피부암 발병률 증가, 광범위한 연구 개발(R&D) 파이프라인, 피부암에 대한 인식 상승 등이 있습니다. 예를 들어, 미국 임상종양학회가 2022년 2월에 업데이트한 자료에 따르면 2020년에 약 324,635명이 흑색종 진단을 받았으며, 2020년에는 미국 내 15-29세 인구에서 약 2,400건의 흑색종 진단을 받은 것으로 추산됩니다. 따라서 인구의 피부암 유병률은 연구 대상 시장 성장을 촉진하고 있습니다.
또한 연구개발 증가에 따라 예측기간 중에 피부암 치료제 수요가 높아질 것으로 예측되고 있습니다.
따라서 위에서 언급 한 요인은 예측 기간 동안 연구 된 시장 성장에 총체적으로 기인합니다. 그러나 치료 및 엄격한 규제 프레임 워크와 관련된 과도한 비용은 예측 기간 동안 시장 성장을 방해 할 것으로 예상됩니다.
피부암 진단 및 치료 시장 동향
암 유형별 비흑색종 부문은 예측 기간 동안 증가할 것으로 예상
비흑색종 피부암은 피부 세포에서 시작되며, 암성(악성) 성장이란 암세포가 주변 조직으로 성장하여 파괴할 수 있는 암세포 그룹을 말합니다. 또한 신체의 다른 부위로 퍼지거나 전이될 수 있지만 비흑색종 피부암에서는 드물게 발생합니다. 따라서 비흑색종 사례가 증가함에 따라 이 부문의 성장을 견인할 것으로 예상됩니다.
팬데믹 기간 동안 팬데믹이 모든 피부암에 미치는 영향을 분석하기 위한 연구가 증가했습니다. 2021년 12월에 발표된 'COVID-19 시대의 치료 지연이 피부암에 미치는 영향: 사례 대조 연구'에 따르면 COVID-19 팬데믹은 피부암 발생률 증가와 관련이 있으며, 봉쇄 이후 기간에 더 많은 피부암 수술이 시행되었으며 이는 비흑색종 암의 일종인 편평 세포 암종(SCC)에만 유의미하다고 언급했습니다. 따라서 팬데믹 기간 동안 진단 및 개선된 치료제에 대한 수요가 증가하여 비흑색종 부문에 긍정적인 영향을 미쳤습니다.
2022년 5월에 업데이트된 피부암 재단 데이터에 따르면 비흑색종 피부암의 약 90%가 태양으로부터의 자외선(UV) 노출과 관련이 있는 것으로 나타났습니다. 피부암 재단의 추정에 따르면 기저세포암(BCC)은 가장 일반적인 형태의 피부암으로, 미국에서 매년 360만 건의 BCC가 진단되는 것으로 추정됩니다. 또한 위에서 언급한 자료에 따르면 미국의 연간 피부암 치료 비용은 81억 달러로 추정되며, 이 중 비흑색종 피부암은 약 48억 달러, 흑색종은 33억 달러에 달한다고 합니다. 따라서 비흑색종 유형의 피부암 발생률과 관련 치료 비용은 미국을 비롯한 전 세계에서 첨단 치료법과 진단에 대한 기회를 창출할 것으로 예상됩니다. 따라서 전체 부문 성장을 촉진할 것으로 예상됩니다.
해당 부문의 R&D 증가에 대한 통찰력도 부문 성장을 견인할 것으로 예상됩니다. 예를 들어, 2021년 12월 노샘프턴 종합병원 NHS 트러스트의 연구원과 방사선 전문의는 특정 유형의 기저세포 또는 편평세포 피부암을 치료하는 데 사용되는 고도로 표적화된 방사선 치료 기술인 스킨 브라키세라피를 도입했습니다.
따라서 비흑색종 부문은 성장하고 있으며 예측 기간 동안 상당한 성장이 예상됩니다. 따라서 조사 대상 시장 성장을 가속합니다.
예측기간 중 북미가 시장을 독점할 것으로 예상
북미는 예측기간을 통해 피부암 및 치료제 시장 전체를 지배할 것으로 예상됩니다. 시장 성장은 피부암의 유병률이나 발생률 증가 등의 요인에 의한 것입니다. 미국은 이 지역에서 가장 큰 시장이 될 것으로 예상됩니다.
암 치료제 연구 개발(R&D)에 중점을 둔 의료 인프라가 잘 구축된 시장 플레이어와 최근 제품 출시 및 미국 내 피부암 부담 증가가 미국 시장의 주요 성장 요인입니다. 예를 들어, 2021년 7월 미국과 캐나다 외 지역에서 MSD로 알려진 Merck는 미국 식품의약국(FDA)으로부터 Merck의 항 PD-1 치료제인 키트루다를 수술이나 방사선으로 치료할 수 없는 국소 진행성 피부 편평 세포 암종(cSCC) 환자의 단독 요법으로 확대 승인받았습니다. 또한, 2022년 5월에는 선도적인 글로벌 생명과학 기업 중 하나인 랩코프(Labcorp.)가 흑색종 치료 옵션에 대한 새로운 분석법을 출시했습니다. 이 새로운 테스트는 종양 조직에서 면역조직화학(IHC)을 통해 림프구 활성화 유전자 3(LAG-3)의 발현 수준을 측정할 수 있습니다. LAG-3는 흑색종 환자에서 임상적 이점이 입증된 면역 종양학 표적입니다. 이 검사는 임상시험과 환자 관리 및 치료 모두에 사용할 수 있습니다. 이러한 최근의 발전은 국내 피부암 진단 및 치료제 수요를 견인하여 아시아 태평양 지역의 전체 시장 성장을 견인할 것으로 예상됩니다.
또한 2022년 5월에 업데이트된 피부암 재단 데이터에 따르면 2022년 미국에서 흑색종 진단 건수는 197,700건으로 추산됩니다. 이 중 97,920건은 표피(피부의 최상층)에 국한된 국소성(비침습성)이며, 99,780건은 표피를 뚫고 피부의 두 번째 층(진피)으로 침투하는 침습성이 될 것으로 예상됩니다. 침습성 사례 중 남성은 57,180건, 여성은 42,600건입니다. 따라서 국내 피부암 발생률과 유병률은 치료를 위한 첨단 진단 및 치료제의 개발을 요구하고 있으며, 이는 이 지역의 전체 시장 성장을 촉진하고 있습니다.
따라서 위의 요인에 따라 미국의 피부암 증례는 진보된 피부암 진단 및 치료 기회를 만들어 국내 시장 전체 성장을 가속할 것으로 예상됩니다.
피부암 진단 및 치료 산업 개요
피부암 진단 및 치료 시장은 세계적으로나 지역적으로 경쟁이 심합니다. 시장은 지속적인 제품 개발 및 출시에 종사하는 여러 주요 기업으로 구성되어 있습니다. 현재 시장을 독점하고 있는 기업으로는 Abbott, Pfizer Inc., Sanofi SA, F. Hoffmann-La Roche Ltd, Labcorp., sun pharmaceuticals industries limited 등을 들 수 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 서포트
목차
제1장 서론
- 조사 전제 조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진 요인
- 피부암 발생률 증가
- 피부암에 대한 의식 확대
- 광범위한 조사 개발
- 시장 성장 억제 요인
- 치료에 따른 고액의 비용
- 엄격한 규제 프레임워크
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체 제품의 위협
- 기업 간 경쟁 정도
제5장 시장 세분화
- 암 유형별
- 흑색종
- 비흑색종
- 유형별
- 진단
- 피부경검사
- 생검
- 유전자 검사
- 기타
- 치료
- 화학요법
- 면역요법
- 표적요법
- 기타
- 진단
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아 태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아 태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 개요
- Abbott
- Amgen, Inc.
- Pfizer Inc.
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- Sanofi
- Merck &Co., Inc.
- Novartis AG
- QIAGEN
- Sun Pharmaceutical Industries Ltd
- Daiichi Sankyo Company, Limited
- Labcorp
- Sirnaomics, Inc.
제7장 시장 기회 및 향후 동향
LYJThe Global Skin Cancer Diagnostics and Therapeutics Market size is estimated at USD 13.82 billion in 2024, and is expected to reach USD 17.82 billion by 2029, growing at a CAGR of 5.21% during the forecast period (2024-2029).
The outbreak of the COVID-19 pandemic had a significant impact on the market studied. After the pandemic began, the World Health Organization (WHO) guidelines suggested that chronic disease patients remain indoors. Hence, this hampered the skin cancer diagnostic market. However, the surge in the number of research for studying the impact of the treatment delay on patients with non-melanoma skin cancer (NMSC) or melanoma cancer amid Covid-19 increased. A study, 'The impact of the Covid-19 pandemic on quality of life in skin cancer patients' published in August 2021, mentioned that due to an immunocompromised/-suppressed status and dependent on the underlying tumor disease and burden, cancer patients might be at an increased risk of developing severe Covid-19 disease and requiring treatment in an intensive care setting. Thus, the increased skin cancer incidences during the pandemic increased the diagnostics for the same and created demand for new advanced therapeutics for treatment. Therefore, the pandemic is predicted to have a positive impact on skin cancer diagnostics and therapeutics during the pandemic phase.
Certain factors driving the market growth include increasing incidence of skin cancer, extensive research and development (R&D) pipelines, and rising awareness about skin cancer. For instance, updated in February 2022 by the American Society of Clinical Oncology, an estimated 324,635 people were diagnosed with melanoma in 2020, and in 2020, about 2,400 cases of melanoma were estimated to be diagnosed in people aged 15 to 29 in the United States. Thus, the prevalence of skin cancer among the population is augmenting the growth of the market studied.
Moreover, the increased R&D is predicted to drive the demand for skin cancer therapeutics over the forecast period. For instance, in January 2022, Immunocore, a commercial-stage biotechnology company pioneering the development of a novel class of T cell receptor (TCR) bispecific immunotherapies designed to treat a broad range of diseases, including cancer, received approval from the United States Food and Drug Administration (FDA) for KIMMTRAK (tebentafusp-tebn) for the treatment of HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma (mUM). Additionally, In April 2022, barnaclanic+ launched its Dermatological Diagnosis Unit for the diagnosis and treatment of skin cancer, and the new unit will also have the latest technology on the market in dermatological diagnosis and skin cancer. Thus, this increasing R&D is expected to drive the growth of the studied market.
Therefore, the factors mentioned above are attributed collectively to the studied market growth over the forecast period. However, the excessive cost associated with therapies and stringent regulatory frameworks is expected to hinder the market growth over the forecast period.
Skin Cancer Diagnostics and Therapeutics Market Trends
Non-Melanoma by Cancer Type Segment is Expected to Grow Over the Forecast Period
Non-melanoma skin cancer starts in skin cells, and a cancerous (malignant) growth is a group of cancer cells that can grow into and destroy nearby tissue. It can also spread (metastasize) to other parts of the body, but this is rare with non-melanoma skin cancer. Thus, the increasing cases of non-melanoma are anticipated to drive the growth of the segment.
The research increased during the pandemic to analyze the impact of a pandemic on all skin cancers. A study 'The impact of treatment delay on skin cancer in COVID-19 era: a case-control study' published in December 2021 mentioned in their discussion that the COVID-19 pandemic is associated with an increased skin cancer incidence and more skin cancer operations were performed in the post-lockdown period which was significant only for squamous cell carcinoma (SCC) a type of non-melanoma cancer. Thus, the demand for diagnostics and improved therapeutics increased during the pandemic, marking a positive impact on the non-melanoma segment.
The Skin Cancer Foundation data updated in May 2022 shows that about 90% of non-melanoma skin cancers are associated with exposure to ultraviolet (UV) radiation from the sun. As per the estimates of the Skin cancer Foundation, basal cell carcinoma (BCC) is the most generic form of skin cancer, and an estimated 3.6 million cases of BCC are diagnosed in the United States each year. And the source mentioned above also reported that the annual cost of treating skin cancers in the United States is estimated at USD 8.1 billion, which is about USD 4.8 billion for non-melanoma skin cancers and USD 3.3 billion for melanoma. Thus, the incidence of non-melanoma type skin cancer and associated treatment costs is anticipated to create opportunities for advanced therapeutics and diagnostics in the United States and other counties across the globe. Thereby, it is expected to boost the overall segment growth.
The insights on increased R&D in the segment are also predicted to drive segment growth. For instance, in December 2021, researchers and radiologists at Northampton General Hospital NHS Trust introduced Skin Brachytherapy, a highly targeted radiotherapy technique used to treat certain types of basal cell or squamous cell skin cancers.
Hence, the non-melanoma segment is growing, and it is expected to have significant growth over the forecast period. Hence, driving the growth of the studied market.
North America is Expected to Dominate the Market Over the Forecast Period
North America is expected to dominate the overall skin cancer and therapeutics market throughout the forecast period. The market growth is due to factors like the increasing prevalence and incidence of skin cancers. The United States is expected to be the largest market in this region.
The well-established healthcare infrastructure-focused market players in research and development (R&D) for cancer therapeutics, coupled with recent product launches and the rising burden of skin cancer in the United States, are primary growth factors for the market in the country. For instance, in July 2021, Merck, known as MSD outside the United States and Canada, received approval from the Food and Drug Administration (FDA) for its expanded label for KEYTRUDA, Merck's anti-PD-1 therapy, as monotherapy for the treatment of patients with locally advanced cutaneous squamous cell carcinoma (cSCC) that is not curable by surgery or radiation. Additionally, in May 2022, Labcorp., one of the leading global life sciences companies, launched a new assay for treatment options for melanoma. The new test enables the measurement of Lymphocyte-activation gene 3 (LAG-3) expression levels by immunohistochemistry (IHC) in tumor tissue. LAG-3 is an immune-oncology target with demonstrable clinical benefits in patients with melanoma. The test is available for use in both clinical trials and the care and treatment of patients. These recent developments are expected to drive the skin cancer diagnostics and therapeutics demand in the country, driving the overall market growth in the region.
Furthermore, the Skin Cancer Foundation data updated in May 2022 shows that an estimated 197,700 cases of melanoma will be diagnosed in the United States in 2022. Among those, 97,920 cases will be in situ (noninvasive), confined to the epidermis (the top layer of skin), and 99,780 cases will be invasive, penetrating the epidermis into the skin's second layer (the dermis). Of the invasive cases, 57,180 will be men and 42,600 women. Thus, the incidence and prevalence of skin cancer in the country are demanding the development of advanced diagnostics and therapeutics in the country for treatment, propelling the overall market growth in the region.
Hence, as per the factors mentioned above, the skin cancer cases in the United States is anticipated to create opportunity for advanced skin cancer diagnostics and therapeutics, driving the overall market growth in the country.
Skin Cancer Diagnostics and Therapeutics Industry Overview
The skin cancer diagnostics and therapeutics market is competitive globally and regionally. The market consists of several major players who are engaged in continuous product development and launches. Some companies currently dominating the market are Abbott, Pfizer Inc., Sanofi SA, F. Hoffmann-La Roche Ltd, Labcorp., and sun pharmaceuticals industries limited, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Incidence of Skin Cancer
- 4.2.2 Rising Awareness About Skin Cancer
- 4.2.3 Extensive Research and Developments
- 4.3 Market Restraints
- 4.3.1 High Cost Associated with Therapy
- 4.3.2 Stringent Regulatory Framework
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Cancer Type
- 5.1.1 Melanoma
- 5.1.2 Non-melanoma
- 5.2 By Type
- 5.2.1 Diagnosis
- 5.2.1.1 Dermatoscopy
- 5.2.1.2 Biopsy
- 5.2.1.3 Genetic Tests
- 5.2.1.4 Others
- 5.2.2 Therapeutics
- 5.2.2.1 Chemotherapy
- 5.2.2.2 Immunotherapy
- 5.2.2.3 Targeted Therapy
- 5.2.2.4 Others
- 5.2.1 Diagnosis
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle-East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle-East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott
- 6.1.2 Amgen, Inc.
- 6.1.3 Pfizer Inc.
- 6.1.4 Bristol-Myers Squibb Company
- 6.1.5 F. Hoffmann-La Roche Ltd
- 6.1.6 Sanofi
- 6.1.7 Merck & Co., Inc.
- 6.1.8 Novartis AG
- 6.1.9 QIAGEN
- 6.1.10 Sun Pharmaceutical Industries Ltd
- 6.1.11 Daiichi Sankyo Company, Limited
- 6.1.12 Labcorp
- 6.1.13 Sirnaomics, Inc.

















