
|
시장보고서
상품코드
1438471
뎅기열 검사 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Dengue Testing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
뎅기열 검사 시장 규모는 2024년 4억 3,619만 달러에 이를 것으로 추정됩니다. 2029년에는 5억 4,020만 달러에 달할 것으로 예측되며, 예측 기간(2024-2029) 동안 4.37%의 연평균 복합 성장률(CAGR)로 성장할 것으로 예상됩니다.

코로나19의 대유행은 뎅기열 검사 시장에 큰 영향을 미쳤습니다. 뎅기열 바이러스와 SARS-CoV-2는 감염 초기에 유사한 증상을 유발합니다. 그 결과, 인구의 상당수가 뎅기열 검사를 받아 시장 성장을 가속했습니다. 예를 들어, 기준연도 6월에 IDR에 게재된 논문에 따르면, 싱가포르에서 감염된 두 명의 환자에서 뎅기열 감염과 코로나19의 임상적, 생화학적 특징이 동일한 것으로 관찰되었습니다. 이 두 사람은 뎅기열 혈청검사 양성으로 뎅기열 진단을 받고 치료를 받았습니다. 그러나 증상이 악화되고 이후 검사에서 코로나19 양성 판정을 받았습니다.
또한, 코로나19가 유행하는 가운데 세계 여러 지역에서 뎅기열 환자가 증가하고 있는 것으로 보고되었습니다. 예를 들어, 기준년 7월에 발표된 기사에 따르면 국내 뎅기열 환자 수가 증가 추세에 있어 이를 억제하기 위해 긴급한 공공 정책이 필요했습니다. 그 결과 검사에 대한 수요가 증가했습니다. 따라서 코로나19는 팬데믹 기간 동안 시장 성장에 큰 영향을 미쳤습니다.
시장 성장에 기여하는 요인으로는 뎅기열 환자 증가와 뎅기열의 위험성을 알리는 캠페인 증가를 꼽을 수 있습니다.
뎅기열의 전반적인 발생률과 폭발적인 유행은 지난 몇 년동안 급격히 증가했습니다. 예를 들어, 유럽 CDC가 발표한 통계에 따르면 올해 전 세계적으로 137만 1,248명의 뎅기열 환자와 849명의 사망자가 보고되었습니다. 자료에 따르면 브라질(1,114,758명)이 가장 많고 페루(45,816명), 베트남(25,694명), 인도네시아(22,331명), 콜롬비아(21,576명)가 그 뒤를 이었습니다. 또한, WHO와 PAHO가 발표한 자료에 따르면, 올해 6월 1일 현재 미국에서는 1,238,528명의 뎅기열 환자가 보고되었고, 544,125명의 환자가 확인되었으며, 426명이 사망했습니다. 이처럼 각국의 뎅기열 환자 부담이 증가함에 따라 뎅기열 검사에 대한 수요가 증가하여 예측 기간 동안 시장 성장을 가속할 것으로 예상됩니다.
또한, 뎅기열 확산을 억제하기 위한 정부 및 의료 전문가들의 노력도 예측 기간 동안 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, 올해 1월 1일부터 4월 30일까지 필리핀에서 854건의 뎅기열 환자를 기록한 지역 역학 및 감시 단위(RESU)에 따라 올해 5월 DoH-CHD는 삭스칼겐에서 뎅기열 캠페인을 강화했습니다.
또한, 뎅기열 검사를 위한 인수, 제휴, 제품 출시 등 다양한 사업 전략을 채택하는 기업들의 관심이 높아지면서 시장 성장에 힘을 실어줄 것으로 예상됩니다. 예를 들어, 기준년 9월 중앙의약품표준관리기구(CDSCO)는 코사라 진단(Cosara Diagnostics)의 SARAGENE 뎅기열 검사 키트의 제조 및 판매를 승인했습니다. 이 검사 키트는 실시간 중합효소 연쇄반응 기술을 기반으로 한 체외 진단용 검사로 코다이애그노스틱스의 특허 기술인 CoPrimers를 사용했습니다. 기준년 3월, 로슈와 덴마크 진단은 로슈가 주당 24.05달러에 전액 현금으로 덴마크 진단을 인수하는 최종 합병 계약을 체결하였습니다.
그러나 효과적인 진단 도구의 부재와 기존 검사 키트의 높은 가격은 예측 기간 동안 시장 성장을 저해할 가능성이 높습니다.
뎅기열 검사 시장 동향
ELISA 기반 검사 부문이 시장에서 큰 점유율을 차지할 것으로 전망
뎅기열 환자 증가, ELISA 기반 뎅기열 검사 도입 확대 등의 요인으로 인해 ELISA 기반 검사 분야가 큰 시장 점유율을 차지할 것으로 예상됩니다.
효소 결합 면역 흡착 분석법(ELISA)은 뎅기열을 진단하는 가장 일반적이고 널리 사용되는 방법 중 하나입니다. 이 검사는 환자의 혈청에서 항 DENV IgM 항체 또는 IgG 항체의 존재를 측정합니다. 뎅기열을 조기에 적시에 진단하고 관리하면 심각한 뎅기열로 인한 이환율과 사망률을 낮추고 더 광범위한 발병의 위험을 줄일 수 있습니다.
표준적인 방법인 ELISA는 NS1 항원을 검출하고 4유형의 뎅기열 바이러스 혈청형을 구별할 수 있습니다. 따라서 뎅기열 검사에서 ELISA의 사용은 예측 기간 동안 증가할 것으로 예상됩니다. 예를 들어, 인도 보건부는 지난 2월 민간 검사 기관과 병원에 ELISA를 통한 확인 검사만 정부 검사 기관에서 실시하도록 지시했습니다.
또한 같은 해 11월에 발표된 연구에 따르면 ANS1 기반 DENV IgG ELISA는 항 DENV 혈청학적 검사의 진단 특이성을 높여 DENV 및 ZIKV가 유행하는 지역에서 혈청학적 분석에 특히 유용할 수 있음을 보여주었습니다. 따라서 뎅기열에 대한 ELISA 기반 검사의 사용은 매년 증가하여 시장 성장을 가속할 것으로 예상됩니다.
또한, 여러 주요 기업이 제공하는 완벽한 검사 키트와 같은 ELISA 플랫폼의 기술적 진보가 시장 성장에 기여하고 있습니다. 예를 들어, Abbott의 Panbio Dengue IGG Indirect ELISA는 뎅기열과 일치하는 임상 증상 및 과거 뎅기열에 노출된 경험이 있는 환자의 실험실 진단을 보조하기 위해 혈청 내 뎅기열 항원 혈청형(1, 2, 3, 4)에 대한 IgG 항체를 검출하는 데 사용됩니다. 검출하는 데 사용됩니다.
따라서 위의 모든 요인을 고려할 때, 이 부문은 예측 기간 동안 꾸준한 성장세를 보일 것으로 예상됩니다.
아시아태평양은 뎅기열 검사 시장에서 가장 빠르게 성장하는 지역입니다.
아시아태평양은 예측 기간 동안 뎅기열 검사 시장에서 가장 빠르게 성장하는 지역이 될 것으로 예상됩니다.
동남아시아 지역의 감염병 확산이 증가하면서 시장 성장에 기여하고 있습니다. 예를 들어, ECDC가 발표한 자료에 따르면 인도네시아에서 약 313건, 스리랑카에서 816건, 동티모르에서 1,286건, 베트남에서 2,440건의 감염 사례가 보고되었습니다. 또한 NCVBDC에 따르면 인도의 뎅기열 환자 수는 기준 연도의 44,585명에서 193,245명으로 증가했습니다. 이 지역의 뎅기열 환자 수가 증가함에 따라 검사에 대한 필요성이 높아져 뎅기열 검사 시장의 성장을 가속할 것으로 예상됩니다.
WHO 데이터에 따르면, 서태평양 지역 말레이시아에서 17,497건의 뎅기열 환자가 보고되었습니다. 이 등록 건수는 기준 연도의 같은 기간에 비해 57.6% 증가한 수치입니다. 이 지역의 뎅기열 감염자 수 증가는 예측 기간 동안 뎅기열 검사 시장의 성장을 가속할 것으로 보입니다.
또한, 코로나19 감염자의 뎅기열 검출에 대한 진단 과제에 대한 논문은 환자가 두 가지 검사에 모두 반응하고 교차 반응성을 보임으로써 코로나19와 뎅기열 바이러스 감염의 상관관계를 보여주었습니다. 이는 뎅기열이 유행하는 국가에서 코로나19 감염을 감지하는 데 있어 공중 보건에 대한 우려를 나타냈습니다.
또한, 기준년도 4월에 발표된 싱가포르에서 뎅기열 바이러스 검출에 사용할 수 있는 6가지 신속 진단 키트의 정확도와 효능을 비교한 연구에서 Standard Q가 뎅기열 바이러스 감염을 검출하는 데 가장 높은 민감도와 특이도를 가진 것으로 나타났습니다. 이 키트는 효율적인 뎅기열 감시를 위해 사용할 수 있습니다. 높은 민감도로 인해 이러한 진단 키트의 필요성이 증가하고 있으며, 이는 예측 기간 동안 시장 성장에 박차를 가할 것으로 보입니다.
또한, 아시아태평양의 여러 지역에서 이러한 전염병이 발생함에 따라 여러 기업이 뎅기열 진단 검사 키트를 개발하여 예측 기간 동안 시장 성장에 기여하고 있습니다. 예를 들어, 아키코는 기준연도 11월에 DNA 앱타머 기술 플랫폼을 적용한 뎅기열 진단키트 개발에 착수했습니다.
정부가 국민들에게 뎅기열에 대한 인식을 확산시키기 위한 노력은 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, 올해 5월 스리랑카 국립 뎅기열 대책본부는 뎅기열 확산을 억제하기 위해 5월 18일부터 5월 24일까지 20개 지역의 위험 지역과 고위험 지역에서 모기 퇴치 프로그램을 시행했습니다.
이러한 요인으로 인해 이 시장은 예측 기간 동안 성장할 것으로 예상됩니다.
뎅기열 검사 산업 개요
뎅기열 검사 시장은 통합되어 있으며, 많은 기업들이 시장 성장에 크게 기여하고 있습니다. 시장 주요 기업으로는 Abbott Laboratories, NovaTec Immundiagnostica GmbH, Euroimmun AG(PerkinElmer), F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc. InBios International Inc. 등이 있습니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 시장 성장 억제요인
- Porter의 Five Forces 분석
- 신규 진출업체의 위협
- 구매자/소비자의 교섭력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계
제5장 시장 세분화
- 제품 유형
- ELISA 기반 검사
- RT-PCR 기반 검사
- 뎅기열 IgG/IgM 신속 검사
- 기타 검사
- 최종사용자
- 병원
- 진단센터
- 기타 최종사용자
- 지역
- 아시아태평양
- 인도
- 필리핀
- 인도네시아
- 말레이시아
- 베트남
- 태국
- 스리랑카
- 기타 아시아태평양
- 남아메리카
- 브라질
- 멕시코
- 니카라과
- 콜롬비아
- 온두라스
- 기타 지역
- 아시아태평양
제6장 경쟁 구도
- 기업 개요
- Abbott Laboratories
- Abnova Corporation
- PerkinElmer Inc(Euroimmun AG)
- Certest Biotec SL
- Diasorin
- F. Hoffmann-La Roche Ltd
- InBios International Inc.
- NovaTec Immundiagnostica GmbH
- OriGene Technologies
- Thermo Fisher Scientific Inc.
- Immundiagnostik AG
- Quest Diagnostics
제7장 시장 기회와 향후 동향
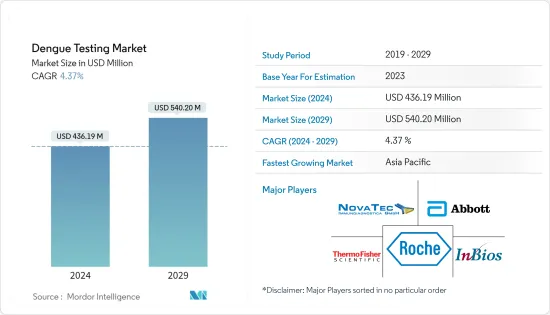
LSH 24.03.18The Dengue Testing Market size is estimated at USD 436.19 million in 2024, and is expected to reach USD 540.20 million by 2029, growing at a CAGR of 4.37% during the forecast period (2024-2029).
The COVID-19 pandemic substantially impacted the dengue testing market. Dengue viruses and SARS-CoV-2 cause similar symptoms in the early stages of infection. Thus, as a result, a significant proportion of the population underwent dengue testing, boosting the market's growth. For instance, as per an article published in IDR in June of the base year, it was observed that the clinical and biochemical characteristics of dengue infection and COVID-19 were identical in two infected patients in Singapore. The two individuals were diagnosed with dengue fever through a positive dengue serological test and were treated for it. However, their symptoms worsened, and a subsequent test revealed that they were COVID-19 positive.
Furthermore, several regions worldwide reported growing dengue cases amid the COVID-19 pandemic. For instance, an article published in July of the base year stated that dengue showed an increasing trend in the number of cases in the country, requiring urgent public policies to curb it. This resulted in an increased demand for testing procedures. Hence, COVID-19 significantly impacted the market's growth during the pandemic.
Certain factors contributing to the market's growth are the increasing incidences of dengue cases and the growth in the number of awareness campaigns to educate about the ills of dengue.
The overall incidence of dengue and the explosive outbreaks of the disease increased dramatically over the last several years. For instance, according to the statistics published by the European CDC, 1,371,248 dengue cases and 849 deaths were reported this year worldwide. In addition, as per the same source, the majority of cases were reported by Brazil (1,114,758), followed by Peru (45,816), Vietnam (25,694), Indonesia (22,331), and Colombia (21,576). Additionally, as per the data published by the WHO and PAHO, as of 1st June this year, there were 1,238,528 dengue cases reported in America, with 544,125 confirmed cases and 426 deaths. Thus, the rising burden of dengue cases across countries is expected to increase the demand for dengue testing, which is anticipated to propel the market's growth over the forecast period.
Similarly, government and healthcare professionals' initiatives to limit dengue spread are expected to boost the market's growth over the forecast period. For instance, in May this year, DoH-CHD ramped up its dengue fever campaign in Soccsksargen after the Regional Epidemiology and Surveillance Unit (RESU) recorded 854 dengue cases in the Philippines from January 1 to April 30 this year.
Moreover, the rising focus of companies on adopting various business strategies, such as acquisition, partnerships, and product launches for dengue testing, is expected to fuel the market's growth. For instance, in September of the base year, the Central Drugs Standard Control Organization (CDSCO) approved Cosara Diagnostics to manufacture and distribute SARAGENE Dengue Test Kit. The test kit is a real-time polymerase chain reaction technology-based in vitro diagnostic test that uses Co-Diagnostics' patented CoPrimers technology. In March of the base year, Roche and GenMark diagnostics entered a definitive merger agreement for Roche to fully acquire GenMark for USD 24.05 per share in an all-cash transaction.
However, the unavailability of effective diagnostic tools and the high price of existing test kits will likely restrain the market's growth over the forecast period.
Dengue Testing Market Trends
The ELISA-based Tests Segment is Anticipated to Hold a Significant Share of the Market
The ELISA-based tests segment is anticipated to hold a significant market share owing to factors such as the increasing incidence of dengue cases and the growing adoption of ELISA-based dengue testing.
Enzyme-linked immunosorbent assay (ELISA) is one of the most common and widely accepted methods for diagnosing dengue. The test measures the presence of anti-DENV IgM or IgG antibodies in the patient's serum. The early and timely diagnosis and management of dengue can reduce the risk of morbidity and mortality rates from severe forms of dengue disease and decrease the risk of wider outbreaks.
ELISA, the standard method, can detect NS1 antigens and differentiate between the four dengue virus serotypes. Thus, the use of ELISA for dengue testing is expected to increase over the forecast period. For instance, in February, the Health Department in India issued directions to private labs and hospitals to get only ELISA confirmatory tests done at government labs, due to which private labs were asked to send samples for the ELISA test if patients test positive for dengue through other initial tests.
Furthermore, a research study published in November of the base year showed that ANS1-based DENV IgG ELISA conferred enhanced diagnostic specificity for anti-DENV serological tests and may be particularly useful for serological analyses in endemic regions for DENV and ZIKV transmission. Thus, using ELISA-based tests for dengue is expected to increase over the years and boost the market's growth.
Moreover, technological advancements in ELISA platforms, such as complete test kits offered by several major players, contribute to the market's growth. For instance, Panbio Dengue IGG Indirect ELISA, a test kit provided by Abbott, is used to detect IgG antibodies to dengue antigen serotypes (1, 2, 3, and 4) in serum as an aid to the clinical laboratory diagnosis of patients with clinical symptoms and past exposure consistent with dengue fever.
Thus, considering all the factors mentioned above, the segment is expected to witness steady growth over the forecast period.
Asia-Pacific is the Fastest Growing Region in the Dengue Testing Market
Asia-Pacific is expected to be the fastest-growing region in the dengue testing market over the forecast period.
The increasing prevalence of disease infections within the region contributes to the market's growth. For instance, as per the data published by the ECDC, approximately 313 cases in Indonesia, 816 cases in Sri Lanka, 1,286 cases in Timor-Leste, and 2,440 cases in Vietnam were reported. Additionally, as per NCVBDC, the number of dengue cases in India increased from 44,585 cases to 193,245 in the base year. A large number of dengue cases in the region, necessitating the requirement of testing, is expected to boost the growth of the dengue testing market.
According to WHO data, 17,497 dengue cases were reported in Malaysia in the Western Pacific region. The registered number reflected a 57.6% increase compared to the same period in the base year. The increasing number of dengue cases in the area will likely propel the growth of the dengue testing market over the forecast period.
Furthermore, an article published about the diagnostic challenges in detecting dengue in COVID-19-affected people showed a correlation between COVID-19 and the dengue virus infection, as the patients were reactive to both tests, indicating a cross-reactivity. This indicated a public health concern in detecting COVID-19 infection in dengue-endemic countries.
Similarly, a study published in April of the base year that compared the accuracy and efficacy of six rapid diagnostic kits available for dengue viral detection in Singapore observed that Standard Q had the highest degree of sensitivity and specificity in detecting dengue virus infection. The kit could be used for efficient dengue disease surveillance. The rise in the necessity of such diagnostic kits, owing to the high sensitivity, is likely to add to the market's growth over the forecast period.
Moreover, such outbreaks across various regions in Asia-Pacific led several companies to develop dengue diagnostic test kits, contributing to the market's growth over the forecast period. For instance, in November of the base year, Achiko initiated the development of a dengue fever diagnostic test by applying its DNA aptamer technology platform.
The initiatives taken by the government to spread dengue awareness among the population are expected to fuel the market's growth. For instance, in May this year, the National Dengue Control Unit of Sri Lanka organized a mosquito control program from 18 May to 24 May in risky and high-risk areas in 20 districts to control the spread of dengue.
Thus, the market is expected to project growth over the forecast period due to the abovementioned factors.
Dengue Testing Industry Overview
The dengue testing market is consolidated, with many companies contributing significantly to the market's growth. Some of the major players in the market are Abbott Laboratories, NovaTec Immundiagnostica GmbH, Euroimmun AG (PerkinElmer), F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc., and InBios International Inc.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increase Incidences of Dengue Cases
- 4.2.2 Increasing Awareness Campaigns to Educate About the Ills of Dengue
- 4.3 Market Restraints
- 4.3.1 Unavailability of Effective Diagnostic Tools
- 4.3.2 High Price of Existing Test Kits
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 Product Type
- 5.1.1 ELISA-based Tests
- 5.1.2 RT-PCR based Tests
- 5.1.3 Dengue IgG/IgM Rapid Test
- 5.1.4 Other Tests
- 5.2 End User
- 5.2.1 Hospitals
- 5.2.2 Diagnostic Centers
- 5.2.3 Other End Users
- 5.3 Geography
- 5.3.1 Asia-Pacific
- 5.3.1.1 India
- 5.3.1.2 Philippines
- 5.3.1.3 Indonesia
- 5.3.1.4 Malaysia
- 5.3.1.5 Vietnam
- 5.3.1.6 Thailand
- 5.3.1.7 Sri Lanka
- 5.3.1.8 Rest of Asia-Pacific
- 5.3.2 Americas
- 5.3.2.1 Brazil
- 5.3.2.2 Mexico
- 5.3.2.3 Nicaragua
- 5.3.2.4 Colombia
- 5.3.2.5 Honduras
- 5.3.2.6 Rest of Americas
- 5.3.1 Asia-Pacific
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Abnova Corporation
- 6.1.3 PerkinElmer Inc (Euroimmun AG)
- 6.1.4 Certest Biotec SL
- 6.1.5 Diasorin
- 6.1.6 F. Hoffmann-La Roche Ltd
- 6.1.7 InBios International Inc.
- 6.1.8 NovaTec Immundiagnostica GmbH
- 6.1.9 OriGene Technologies
- 6.1.10 Thermo Fisher Scientific Inc.
- 6.1.11 Immundiagnostik AG
- 6.1.12 Quest Diagnostics













