
|
시장보고서
상품코드
1439807
경피 흡수형 피부 패치 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Transdermal Skin Patches - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
경피 흡수형 피부 패치 시장 규모는 2024년 89억 2,000만 달러에 이를 것으로 추정됩니다. 2029년에는 113억 3,000만 달러에 달할 것으로 예측되며, 예측 기간(2024-2029년) 동안 4.87%의 연평균 복합 성장률(CAGR)을 나타낼 것으로 예상됩니다.

코로나19의 발생은 경피흡수성 피부 패치 시장에 영향을 미치고 있습니다. 이 패치는 정의된 피부 미세환경에 안전하고 반복적으로 제어된 약물 투여를 가능하게 합니다. 이에 따라, 코로나19 백신 가능성을 연구하는 연구자들은 손가락 끝 크기의 피부 패치를 사용하여 투여하는 것을 고려하고 있습니다. 예를 들어, 2020년 5월에 발표된 논문에 따르면, 피츠버그 대학 의료센터와 피츠버그 대학 연구진은 스파이크 단백질 조각을 피부에 주입할 수 있는 400개의 미세 바늘이 있는 손가락 끝 크기의 패치인 마이크로니들 어레이(MNA)를 개발하려고 시도했다고 합니다.
또한 2021년 1월, 스완지대학교 혁신소재, 가공 및 수치기술연구소(IMPACT) 연구팀은 마이크로니들(MN)을 이용해 코로나19 스마트 백신 패치를 제작했습니다. 이 프로젝트의 주요 목표는 코로나19 백신을 경피적으로 투여할 수 있을 뿐만 아니라, 저침습적인 방법으로 피부 구획 내 바이오마커를 모니터링하고 백신 접종 효과에 대한 실시간 정보를 제공할 수 있는 스마트 백신 전달 장치의 프로토타입을 제작하는 것이었습니다. 하는 것이었습니다. 따라서 COVID-19는 조사 대상 시장에 큰 영향을 미치고 있습니다.
시장 성장의 요인으로는 경피약이 경구약이나 경구 섭취약에 비해 경피약의 장점 증가, 의약품 개발에 대한 자금 지원 및 투자 증가를 들 수 있습니다.
경구용 약은 메스꺼움이나 배탈과 같은 소화기 부작용이 있을 수 있습니다. 그러나 경피 패치는 일반적으로 소화기 부작용을 예방할 수 있습니다. 예를 들어, 2022년 2월에 발표된 논문에 따르면 경피 패치는 소화관을 우회하여 잠재적인 부작용을 피하고 피부를 통해 혈류에 직접 약물을 주입하는 것으로 확인되었습니다. 따라서 경피 패치는 비침습적이고 통증이 없는 약물 투여 방법이며, 지정된 기간 동안 지속적으로 치료 용량을 투여할 수 있다는 장점도 있습니다. 이 때문에 대상자들 사이에서 채택이 증가하여 시장 성장을 가속할 것으로 예상됩니다.
이러한 패치는 편두통, 호르몬, 통증, 심혈관 질환, 신경 질환, 금연 치료에도 사용됩니다. 현재 경피적 약물 전달 시스템은 투여 횟수 감소, 생체 이용률 향상, 부작용 감소, 패치 제거를 통해 언제든지 약물 투입을 중단할 수 있다는 장점으로 인해 수요가 증가하고 있습니다.
또한, 전 세계적으로 담배 사용량이 증가하여 건강 관리의 부담이 되고 있습니다. 예를 들어, WHO가 2022년 5월에 발표한 데이터에 따르면 2021년에는 전 세계 인구의 22.3%가 담배를 사용하고 있으며, 그 중 남성은 전체 인구의 36.7%, 여성은 전 세계 여성 인구의 7.8%를 차지하는 것으로 확인되었습니다. 따라서 전 세계 흡연자가 증가함에 따라 니코틴 경피흡수성 피부 패치 사용과 환자 수가 증가함에 따라 조사 대상 시장을 견인할 것으로 예상됩니다.
또한, 경피 패치 시장에 진입하기 위해 제휴, 인수, 제품 출시 등 다양한 비즈니스 전략을 채택하는 성장 기업의 관심도 시장 성장에 기여하고 있습니다. 예를 들어, 2021년 12월 Luye Pharma Group은 자회사 Luye Pharma Switzerland AG가 중국 Changchun GeneScience Pharmaceutical(Gensci)과 중국 본토에서 리바스티그민 단회용 경피 패치(리바스티그민)를 판매하기로 합의했습니다. 리바스티그민 단일 경피 패치(리바스티그민 SD) 및 리바스티그민 다일 경피 패치(리바스티그민 MD)에 대한 중국 내 독점적 상업화 권리를 부여했다고 발표했습니다. 또한 2021년 9월, 임퀘스트 바이오사이언스는 항레트로바이러스(ARV) 경피 전달 패치를 출시했습니다. 이 장치는 고분자 패치와 필름을 이용해 7일에 걸쳐 약물을 피부로 방출합니다.
또한, 사람들의 건강에 대한 인식이 높아지고 소비 가능한 소득이 증가함에 따라 의료에 대한 지출도 증가하고 있습니다. 또한 각국 정부는 의약품 연구에 많은 투자를 하고 있습니다. 예를 들어, 2021년 10월 미국 식품의약국(USFDA)은 희귀질환 치료를 위한 신약 개발 임상시험에 11개의 보조금을 수여했습니다. 또한, 2022년 1월 캐나다 정부가 발표한 자료에 따르면, 공공 부문은 2,262억 4,650만 달러, 민간 부문은 752억 8,300만 달러를 의약품 개발에 투자한 것으로 나타났습니다. 이러한 정부 투자는 경피흡수형 의약품과 패치제 시장의 기회를 창출하고 시장 성장을 가속할 것으로 예상됩니다.
그러나 피부는 다양한 활성 물질을 흡수할 수 없기 때문에 예측 기간 동안 시장 성장에 걸림돌이 될 것으로 예상됩니다.
경피흡수형 피부패치 시장 동향
통증 완화 부문은 예측 기간 동안 경피 흡수형 피부 패치 시장에서 높은 CAGR을 기록할 것으로 예상
통증 완화 부문은 예측 기간 동안 경피 패치 시장에서 큰 성장을 보일 것으로 예상됩니다.
이 부문 성장의 요인은 당뇨병성 신경병증, 류마티스 관절염, 골관절염, 편두통과 같은 통증 관련 질환의 유병률이 인구 사이에서 증가하고 있기 때문입니다. 예를 들어, 2022년 2월에 발표된 기사에 따르면 요통은 성인들에게 흔한 질병이며, 전 세계 총 인구의 최대 23%가 만성 요통을 경험하고 있는 것으로 관찰되었습니다. 또한, 해당 출처에 따르면 대상자의 1년 후 재발률은 24%에서 80%까지이며, 평생 유병률은 성인 인구의 84%로 추정됩니다. 이처럼 인구 중 요통의 부담이 증가함에 따라 통증 완화 패치에 대한 수요가 증가하여 부문의 성장을 가속할 것으로 예상됩니다.
또한 2021년 업데이트된 편두통 연구 재단의 데이터에 따르면 편두통은 미국에서 약 3,900만 명의 남성, 여성, 어린이가 앓고 있으며, 전 세계적으로 10억 명이 앓고 있는 매우 유병률이 높은 신경 질환입니다. 편두통은 세계에서 세 번째로 흔한 질병으로 간주되며, 편두통 통증 완화에 효과적인 패치에 대한 수요가 증가하여 시장 성장을 가속할 것으로 예상됩니다.
또한, 통증 완화 패치 개발에 대한 기업 활동 증가도 예측 기간 동안 시장 성장에 기여하고 있습니다. 예를 들어, 2021년 11월 NEXGEL은 NEXGEL의 독자적인 하이드로겔 기술을 사용하여 개발된 MEDAGEL 편두통 완화 패치를 출시했습니다. 이 패치는 몸에서 열을 빼내어 편두통, 호르몬성 두통, 발열을 즉각적으로 장시간 냉각 완화합니다.
이러한 요인으로 인해 이 부문은 예측 기간 동안 크게 성장할 것으로 예상됩니다.
북미가 시장을 지배하고 있으며, 예측 기간 동안에도 계속 지배할 것으로 예상
북미는 예측 기간 동안 경피 패치 시장이 크게 성장할 것으로 예상됩니다.
시장 성장의 요인은 주요 기업의 존재와 잘 구축된 의료 인프라입니다. 또한, 정부 이니셔티브 증가와 연구 제휴 증가도 시장 성장에 기여하고 있습니다.
북미에서는 미국이 경피용 패치 시장에서 가장 큰 점유율을 차지할 것으로 예상됩니다. 이는 의료 정책의 지원, 통증 및 기타 만성 질환을 앓고 있는 환자가 많고 의료 시장이 발달했기 때문입니다.
이 지역의 흡연자 수 증가는 니코틴 경피용 패치에 대한 수요를 증가시켜 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, CDC가 2022년 3월 발표한 자료에 따르면, 고등학생의 약 34.0%(522만 명), 중학생의 약 11.3%(134만 명)가 담배 제품(전자담배, 시가, 파이프 담배, 가열식 담배 제품, 니코틴 파우치, 비디 등)을 사용해 본 적이 있다고 응답했습니다. 담배 등)을 사용한 적이 있다고 응답했습니다. 또한, 고등학생과 중학생은 전자담배가 가장 많이 사용하는 담배 제품이라는 응답이 가장 많았고, 담배, 시가, 무연담배, 갈고리 담배, 니코틴 파우치, 가열식 담배 제품, 파이프 담배가 그 뒤를 이었습니다.
캐나다 폐협회(Canadian Lung Association 2021)에 따르면, 담배는 캐나다에서 예방 가능한 질병과 사망의 주요 원인입니다. 매년 약 48,000명의 캐나다인이 흡연으로 인해 사망하는 것으로 추정됩니다. 기타에도 수많은 사람들이 장기적인 질병을 앓고 있습니다. 공중보건 교육 및 예방 노력에도 불구하고 캐나다인의 약 15%가 흡연을 계속하고 있으며, 그 결과 약물 보급률이 증가하고 있습니다. 경피용 패치와 같은 혁신적인 신약의 등장은 시장 성장을 가속화하고 있습니다.
또한, 유방암과 전립선암 등 암 발병률이 증가하고 있는 것도 시장 성장을 크게 가속화할 것으로 보입니다. 예를 들어, GLOBOCAN 2020 보고서에 따르면 2020년 멕시코에서 보고된 암 환자는 19만 5,499명이며, 그 중 유방암과 전립선암이 가장 많은 것으로 나타났습니다. 또한 GLOBOCAN 2020에 따르면 멕시코의 암 발병률은 2030년까지 25만 4,665건, 2040년까지 32만 3,432건으로 증가할 것으로 예측됩니다. 이처럼 인구의 암 발병률이 증가함에 따라 암 치료로 인한 통증 완화를 위한 약물 나노입자를 이용한 경피용 패치에 대한 수요가 증가하여 시장 성장을 가속할 것으로 예상됩니다.
또한, 멕시코에서는 정부 당국과 기타 조직이 흡연을 방지하기 위해 다양한 이니셔티브를 취하고 있으며, 이는 시장 성장을 지원할 가능성이 높습니다. 예를 들어, 2022년 4월, 칸쿤은 미국 관광객을 대상으로 한 새로운 캠페인을 시작했으며, 현지 당국은 CGC(종합 커뮤니케이션 조정)와 협력하여 외국인 관광객을 대상으로 'Be part of the solution'이라는 제목의 캠페인을 실시했습니다. 라는 제목의 캠페인을 진행했습니다. 따라서 이러한 노력은 개인들 사이에서 인식을 조성하고 국내 경피 흡수형 피부 패치에 대한 수요를 촉진하고 있습니다.
또한, 각 기업이 시장 지위를 유지하기 위해 채택한 제품 출시 및 사업 전략 증가도 시장 성장에 기여하고 있습니다. 예를 들어, 2021년 4월 BASF는 정서적 행복감을 향상시키는 새로운 스킨케어 활성 성분인 'Sacred Patch'를 출시했습니다. 마찬가지로 2020년 Amneal Pharmaceuticals Inc.는 부트란(부프레노르핀) 경피 시스템의 제네릭 의약품인 5mcg/hr, 7.5mcg/hr, 10mcg/hr, 15mcg/hr, 20mcg/hr에 대한 약식 신약 허가 신청(ANDA)을 미국 식품의약국(USFDA)으로부터 취득하였습니다.
이러한 요인으로 인해 예측 기간 동안 시장은 크게 성장할 것으로 예상됩니다.
경피흡수형 피부패치 산업 개요
경피 흡수형 테이프 제제 시장의 경쟁은 중간 정도입니다. 주요 기업들은 경쟁 환경을 유지하기 위한 개발 전략으로 제품 혁신, 제품 출시 및 승인, 경피 패치 발전을 위한 R&D 투자, M&A를 채택하는 데 주력하고 있습니다. 시장 주요 기업으로는 Teva Pharmaceuticals USA Inc., Novartis AG, Teikoku Pharma USA Inc, Mylan Inc. 등이 있습니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 시장 성장 억제요인
- Porter의 Five Forces 분석
- 신규 진출업체의 위협
- 구매자/소비자의 교섭력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계
제5장 시장 세분화
- 유형별
- Single-layer Drug-in-Adhesive
- Multi-layer Drug-in-Adhesive
- Matrix
- 기타 유형
- 용도별
- 통증 완화
- 금연 보조
- 과민성 방광
- 호르몬 요법
- 기타 용도
- 지역
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 개요
- Teva Pharmaceutical Industries Ltd
- Novartis AG
- Teikoku Pharma USA Inc.(Teikoku Seiyaku Co. Ltd)
- Viatris Inc.
- Johnson & Johnson
- Luye Pharma Group
- Purdue Pharma Manufacturing LP
- Henan Lingrui Pharmaceutical Ltd
- Samyang Biopharmaceuticals Corp.(Samyang Holdings)
제7장 시장 기회와 향후 동향
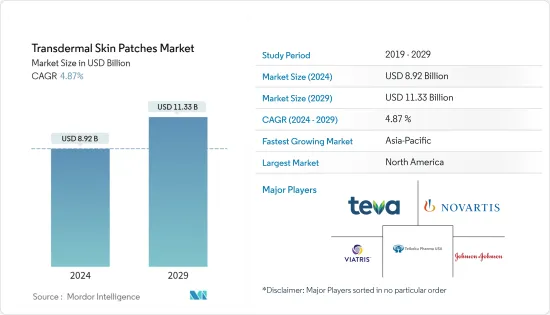
LSH 24.03.19The Transdermal Skin Patches Market size is estimated at USD 8.92 billion in 2024, and is expected to reach USD 11.33 billion by 2029, growing at a CAGR of 4.87% during the forecast period (2024-2029).
The COVID-19 outbreak has impacted the transdermal skin patches market. These patches enable safe, reproducible, and controlled administration of drugs to a defined skin microenvironment. Therefore, researchers working on a potential vaccine against the new COVID-19 strain explored the uses of a fingertip-sized skin patch for delivery. For instance, an article published in May 2020 stated that researchers at the University of Pittsburgh Medical Center and the University of Pittsburgh attempted to develop a microneedle array (MNA), a fingertip-sized patch with 400 microscopic needles that could inject spike protein fragments into the skin.
Additionally, in January 2021, a team at the Swansea University Institute for Innovative Materials, Processing, and Numerical Technologies (IMPACT) produced the COVID-19 smart vaccine patch using microneedles (MNs). The primary goal of this project was to create a prototype of the smart vaccine delivery device that could not only deliver the COVID-19 vaccine transdermal but also monitor biomarkers in the skin compartment in a minimally invasive way, offering real-time information on the efficacy of the vaccination. Hence, COVID-19 has a significant impact on the studied market.
Certain factors attributing to the market growth are the increasing advantages of transdermal medicine over oral and ingesting medications and increasing funding and investments in drug development.
Oral medications can have gastrointestinal adverse effects like nausea or upset stomach. However, digestive side effects can usually be prevented with transdermal patches. For instance, according to an article published in February 2022, it has been observed that the transdermal patch delivers medication directly to the bloodstream through the skin, bypassing the digestive tract and any potential side effects. Transdermal patches, therefore, offer a non-invasive, painless method of drug administration with the added benefit of delivering a continuous therapeutic dose for a specified period. This is expected to increase its adoption among the target population, propelling market growth.
These patches also treat migraine, hormones, pain, cardiovascular diseases, neurology disease, and smoking cessation. Currently, the demand for the transdermal route of the drug delivery system is increasing due to decreased dose frequency, greater bioavailability, reduced side effects, and drug input cessation at any time by removing the patch.
Additionally, the increasing use of tobacco in the form of cigarettes has increased globally over time, creating a healthcare burden. For instance, as per the data published by the WHO data in May 2022, it has been observed that 22.3% of the global population used tobacco in 2021, of which men accounted for 36.7% of the total male population, and women accounted for 7.8% of the world's women population. Therefore, with the increasing number of smokers globally, the usage of nicotine transdermal skin patches and the rising number of patients is expected to boost the market studied.
Furthermore, the growing company's focus on adopting various business strategies such as collaborations, acquisitions, product launches, and others to enter the transdermal patches market contributes to market growth. For instance, in December 2021, Luye Pharma Group announced that their subsidiary Luye Pharma Switzerland AG has agreed with Changchun GeneScience Pharmaceutical Co. Ltd (Gensci) of China, under which the company grants Gensci the exclusive commercialization rights of Rivastigmine Single-Day Transdermal Patch (Rivastigmine SD) and Rivastigmine Multi-Day Transdermal Patch (Rivastigmine MD) on the Chinese mainland. Additionally, in September 2021, ImQuest Biosciences launched its antiretroviral (ARV) transdermal delivery patch. The device utilizes a polymeric patch and film, which releases the drug into the skin over seven days.
Moreover, the increasing awareness among the people for their health and the increasing expendable income leads to high expenditure on healthcare. Also, governments across the globe are investing heavily in drug research. For instance, in October 2021, USFDA awarded 11 grants to clinical trials to develop new medical products for rare disease treatments. Additionally, from the data published by the Canadian government in January 2022, it was found that the public and private sectors invested USD 226,246.5 million and USD 75,208.3 million in developing pharmaceutical drugs, respectively. Such government investments are expected to create opportunities for transdermal medicines or patches, thereby boosting the market growth.
However, the inability of the skin to absorb a range of active substances is expected to hinder the market growth over the forecast period.
Transdermal Skin Patches Market Trends
Pain Relief Segment Expects to Register a High CAGR in the Transdermal Skin Patches Market Over the Forecast Period
The pain-relief segment is expected to witness significant growth in the transdermal patches market over the forecast period.
The factors attributing to the segment growth are the rising prevalence of pain-related disorders, such as diabetic neuropathy, rheumatoid arthritis, osteoarthritis, migraine, and other diseases among the population. For instance, from an article published in February 2022, it was observed that back pain is a common ailment among adults and up to 23% of the total population of the world experience chronic low back pain. In addition, as per the same source, the target group also revealed a one-year recurrence rate of 24% to 80%, and some estimates of lifetime prevalence are as high as 84% in the adult population. Thus, the rising burden of back pain among the population is anticipated to increase the demand for pain relief patches, thereby bolstering segment growth.
Furthermore, according to the Migraine Research Foundation data updated in 2021, migraine is an extraordinarily prevalent neurological disease affecting about 39 million men, women, and children in the United States and 1 billion globally. Migraine is considered the third-most prevalent illness in the world, which is expected to raise the demand for effective patches that helps in relieving pain from migraine headaches, thereby propelling the market growth.
Moreover, the rising company activities in developing pain relief patches also contribute to the market growth over the forecast period. For instance, in November 2021, NEXGEL launched its MEDAGEL Migraine Relief Patch, developed using NEXGEL's unique hydrogel technology. The patch provides instant and long-lasting cooling relief from migraines, hormonal headaches, and fevers by pulling heat away from the body.
Thus, owing to the factors mentioned above, the segment is expected to grow significantly during the forecast period.
North America Dominates the Market and is Expected to Continue Dominating During the Forecast Period
North America is expected to witness significant growth in the transdermal patches market over the forecast period.
The factors attributing to the market growth are the presence of key players and established healthcare infrastructure. In addition, the rising government initiatives and an increase in the number of research partnerships are also contributing to the market growth.
In North America, the United States is expected to hold the maximum share of the transdermal patches market due to supportive healthcare policies, many patients suffering from pain and other chronic diseases, and a developed healthcare market.
The rising number of smokers in the region is expected to increase the demand for nicotine transdermal patches, which is expected to increase market growth. For instance, from the data published by CDC in March 2022, it has been observed that about 34.0% of high school students (5.22 million) and 11.3% of middle school students (1.34 million) reported ever using a tobacco product (i.e., electronic cigarettes [e-cigarettes], cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, heated tobacco products, nicotine pouches, and bidis [small brown cigarettes wrapped in a leaf. Additionally, e-cigarettes are the most often used tobacco product from the same source, followed by cigarettes, cigars, smokeless tobacco, hookahs, nicotine pouches, heated tobacco products, and pipe tobacco among high school and middle school students.
According to the Canadian Lung Association 2021, tobacco is the leading cause of preventable disease and death in Canada. Each year, an estimated 48,000 Canadians die because of smoking. Countless others suffer from long-term illnesses. Despite public health education and preventive efforts, about 15% of Canadians continue to smoke, which has increased the widespread availability of drugs. The advent of new innovative drugs, such as transdermal patches, has accelerated the market's growth.
Additionally, the rising incidence of cancers, including breast and prostate cancer, is probably going to accelerate market growth greatly. For instance, according to the GLOBOCAN 2020 report, 195,499 cancer cases were reported in Mexico in 2020, with breast and prostate cancers being the most common among the population. Furthermore, according to Globocan 2020, the incidence of cancer in Mexico is predicted to rise to 254,665 cases by 2030 and 323,432 cases by 2040. Thus, the growing burden of cancer among the population is expected to increase the demand for transdermal patches with drug nanoparticles that relieve pain from cancer therapy, propelling the market growth.
Furthermore, various initiatives taken by the government authority and other organizations in Mexico to prevent smoking are likely to support the market's growth. For instance, in April 2022, Cancun launched a new campaign aimed at United States visitors. In collaboration with the General Communication Coordination (CGC), local authorities aimed toward international tourists dubbed "Be a part of the solution. ". Thus, such initiatives are created awareness among individuals and drive the demand for transdermal skin patches in the country.
Moreover, the rising product launches and business strategies adopted by the companies to withhold their market position also contribute to market growth. For instance, in April 2021, BASF launched 'Sacred Patch,' a new active skincare ingredient that could help boost emotional well-being. Similarly, in 2020, Amneal Pharmaceuticals Inc. received the Abbreviated New Drug Application (ANDA) approval from the USFDA for a generic version of Butrans (buprenorphine) Transdermal System, 5 mcg/hr, 7.5 mcg/hr, 10 mcg/hr, 15 mcg/hr, and 20 mcg/hr.
Thus, owing to the factors mentioned above, the market is expected to grow significantly during the forecast period.
Transdermal Skin Patches Industry Overview
The transdermal skin patches market is moderately competitive. The key players are focused on adopting product innovations, product launches and approvals, R&D investment for advancements in transdermal patches, and mergers and acquisitions as their developmental strategies to sustain the competitive market environment. Some major companies in the market are Teva Pharmaceuticals USA Inc., Novartis AG, Teikoku Pharma USA Inc., Mylan Inc., and 3M.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Advantages of Transdermal Medicine Over Oral and Ingesting Medications
- 4.2.2 Increasing Funding and Investments in Drug Research
- 4.3 Market Restraints
- 4.3.1 Inability of the Skin to Absorb a Range of Active Substance
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Type
- 5.1.1 Single-layer Drug-in-Adhesive
- 5.1.2 Multi-layer Drug-in-Adhesive
- 5.1.3 Matrix
- 5.1.4 Other Types
- 5.2 By Application
- 5.2.1 Pain Relief
- 5.2.2 Smoking Reduction and Cessation Aid
- 5.2.3 Overactive Bladder
- 5.2.4 Hormonal Therapy
- 5.2.5 Other Applications
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East & Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East & Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Teva Pharmaceutical Industries Ltd
- 6.1.2 Novartis AG
- 6.1.3 Teikoku Pharma USA Inc. (Teikoku Seiyaku Co. Ltd)
- 6.1.4 Viatris Inc.
- 6.1.5 Johnson & Johnson
- 6.1.6 Luye Pharma Group
- 6.1.7 Purdue Pharma Manufacturing LP
- 6.1.8 Henan Lingrui Pharmaceutical Ltd
- 6.1.9 Samyang Biopharmaceuticals Corp. (Samyang Holdings)

















