
|
시장보고서
상품코드
1440178
세계 의약품 분석 시험 시장 : 점유율 분석, 업계 동향과 통계, 성장 예측(2024-2029년)Pharmaceutical Analytical Testing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
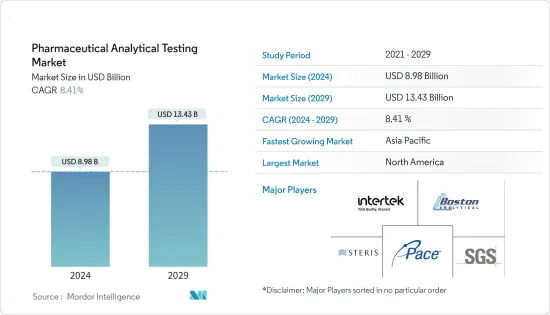
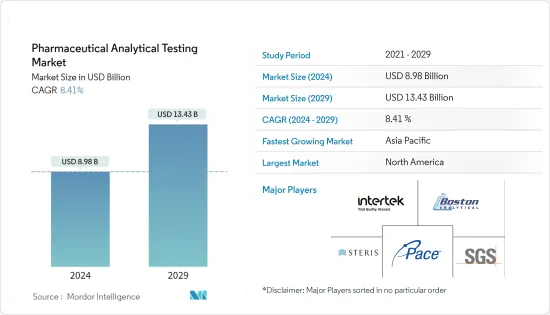
의약품 분석 시험 시장 규모는 2024년에 89억 8,000만 달러로 추정되고, 2029년까지 134억 3,000만 달러에 이를 것으로 예측되며, 예측 기간 동안 복합 연간 성장률(CAGR) 8.41%로 성장할 전망입니다.
COVID-19의 확산을 억제하기 위한 신약 후보와 백신 개발 수요가 증가함에 따라 신형 코로나 바이러스 감염이 의약품 분석 시장에 미치는 영향은 중대했습니다. 따라서 정확성, 효율성 및 안전성을 모니터링하기 위해 의약품 개발의 모든 단계에서 분석 테스트가 필요합니다. COVID-19 감염의 통제 불가능하고 급격한 증가는 바이오시밀러, 복합 분자 및 기타 혁신적인 백신 및 의약품의 개발을 증가시켜 전기영동, 전기화학, 적정 분석 등의 중요한 생물 분석 검사 수요가 증가하고 있습니다. 그리고 면역 분석.
또한, 임상시험 수 증가, 생물제제 및 바이오시밀러의 분석시험의 초점, 임상검사서비스의 아웃소싱 경향 증가가 조사 대상 시장의 성장에 적극적인 영향을 미치고 있습니다.
FDA의 의약품 리콜 통계에 의하면, 세계 매년 약 1,279의 의약품이 리콜되고 있으며, FDA의 의약품 리콜의 94%가 미국에서 발생하고(2021년)에는 캐나다에서의 FDA 의약품 리콜의 4%가 계속 합니다. FDA는 미국에서 12,028건의 의약품 리콜을 발행했습니다. 이 때문에 제품을 시장에 내놓기 전에 제품을 테스트하고 공중의 안전을 확보할 필요성이 떠오르고 있습니다. 의약품의 품질이 낮으면 환자의 건강과 자금제공 시스템에 영향을 미치기 때문에 의약품은 인간과 동물의 더 나은 결과를 목적으로 합니다. 따라서 분석 시험은 의약품의 안전성과 효능을 보장하는데 중요한 역할을 합니다.
R&D 활동, 협업, 전략적 파트너십 증가로 시장 성장이 촉진될 것으로 예상됩니다. 예를 들어(2021년) 11월에 Alcami Corporation, Inc.는 Novavax와 마스터 랩 서비스 계약을 체결했습니다. 이 계약에 따라 Novavax는 Matrix-M 보조제를 포함한 재조합 나노입자 단백질 기반 COVID-19 백신 후보에 대한 분석 시험 지원을 제공하기 위한 풀타임 상당(FTE) 자원을 확보했습니다. 이러한 발전은 시장 성장을 가속할 것으로 추정됩니다.
따라서 앞서 언급한 요인들로 인해 조사 대상 시장은 분석 기간 동안 성장할 것으로 예상됩니다. 그러나 연구소를 유지하기 위한 복잡한 규제 프레임워크와 적절한 분석 기술 개발의 어려움은 시장 성장을 방해할 수 있습니다.
의약품 분석 시험 시장 동향
안정성 테스트 부문은 예측 기간 동안 상당한 시장 성장을 나타낼 것으로 예상
안정성 시험은 약물이 유효 기간 동안 그 특성을 유지하는 능력을 평가하기 위해 수행됩니다. 의약품 및 약물의 안정성 연구는 신약 및 새로운 제제 개발에 가장 중요한 매개 변수 중 하나입니다. 안정성 시험은 화학적, 물리적, 미생물학적, 치료적, 독성의 5가지 파라미터에 기초하여 수행됩니다. 이러한 매개 변수 중 하나에 대한 열화는 건강 피해를 유발할 수 있습니다.
만료일 예측은 모든 제형의 의약품 개발에 필수적입니다. 또한 보관 조건을 결정하고 라벨의 지시를 제안하는 데에도 사용됩니다. 의약품의 안정성 시험은 의약품의 수락 및 승인 전제조건으로 간주되는 제품의 품질, 안전성 및 유효 기간 동안 효능을 유지합니다. 그리고 이러한 연구는 세계 보건기구의 지침을 따라야합니다. Clinicaltrial.gov의 2022년 최신 정보에 따르면, 올해까지 433,207건의 연구가 등록되었으며, 그 중 53%가 미국 외에 등록되었으며, 31%가 미국에 등록되었습니다. 따라서 이러한 요인들은 부문의 성장을 가속할 것으로 예상됩니다.
제품 출시, 승인, 파트너십 등 주요 기업의 전략적 노력도 이 부문의 성장을 가속하고 있습니다. 예를 들어 LGM Pharma는 2021년 7월에 제약 약국을 포함한 의약품 개발자 및 제조자를 위한 분석 시험 및 안정성 서비스를 시작했습니다. 이러한 발전은 예측 기간 동안 부문의 성장을 가속할 것으로 추정됩니다.
북미는 시장에서 큰 점유율을 차지하고 있으며 예측기간 동안에도 마찬가지로 성장할 것으로 예상
북미는 R&D 활동과 투자 증가로 의약품 분석 시험 시장에서 지배적인 지역이 될 것으로 예상됩니다.
식품의약국(FDA) 의약품평가연구센터(CDER)가 제공한 데이터에 따르면 2020년에는 약 53개의 신약이 승인되었으며(2021년)에는 50개의 신약이 승인되었습니다. 2020년과 2021년에 세계적으로 승인된 의약품의 합계 중 대부분은 FDA에 따라 세계 어느 나라보다 먼저 미국에서 승인되었습니다. 또한 임상시험에 관해서는 미국에서는 올해까지 136,276건의 임상시험이 등록되어 있으며, 등록된 임상시험 전체의 31%를 차지하고 있습니다. 이 지역에서의 의약품 승인 빈도의 높이는 예측 기간 동안 이 지역 시장 성장을 가속할 것으로 추정됩니다.
게다가 NIH에 따르면 대통령의 예산은 우선순위가 높은 바이러스 패밀리에 대한 백신, 진단약, 치료제의 연구개발, 바이오세이프티, 바이오보안 등 팬데믹에 대한 대비를 지원하기 위한 필수 자원으로서 2023년도의 121억 달러가 포함되어 있다고 합니다. 미국의 검사 능력과 임상시험 인프라. 이러한 임상시험의 수, 의약품의 승인수 증가, 연구개발비 증가에 대한 대기업의 참여는 북미에서의 의약품 분석검사 시장의 성장 수요가 높아질 것으로 예상됩니다.
또한 제품 출시, 승인, 파트너십 등 주요 기업의 전략적 노력도 이 부문의 성장을 가속하고 있습니다. 예를 들어(2021년) 12월, Pace Science and Technology Company의 부서인 Pace Analytical Services는 업계의 능력을 강화하기 위해 Special Pathogens Laboratory를 인수했습니다. 이러한 발전은 이 지역 시장 성장을 가속할 것으로 추정됩니다.
의약품 분석 시험 업계 개요
의약품 분석 시험 시장은 세계에 수많은 기업이 존재하기 때문에 본질적으로 세분화되어 있습니다. 조사 대상 시장은 Laboratory Testing Inc., Eurofins Scientific, SGS SA, Toxikon Inc., Pace Analytical Services 등 시장 점유율의 대부분을 보유하고 있으며 잘 알려진 여러 국제 및 지역 기업으로 구성됩니다. 되었습니다. Intertek Pharmaceutical Services, Boston Analytical, West Pharmaceutical Services Inc., Steris.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 임상시험수 증가
- 생물제제 및 바이오시밀러의 분석시험에 초점을 맞춘
- 임상 검사 서비스의 아웃소싱 증가 추세
- 시장 성장 억제요인
- 연구소를 유지하기 위한 복잡한 규제 프레임워크
- 적절한 분석 기술 개발의 과제
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체 제품의 위협
- 경쟁 기업간 경쟁 관계의 격렬
제5장 시장 세분화
- 서비스 유형별
- 생체분석검사
- 방법 개발 및 검증
- 안정성 시험
- 원약검사
- 기타 서비스 유형
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Laboratory Testing Inc.
- Eurofins Scientific
- SGS SA
- Labcorp(Toxikon Inc)
- Pace Analytical Services
- Intertek Phamaceutical Services
- Boston Analytical
- West Pharmaceutical Services Inc.
- Steris
제7장 시장 기회와 미래 동향
BJH 24.03.11The Pharmaceutical Analytical Testing Market size is estimated at USD 8.98 billion in 2024, and is expected to reach USD 13.43 billion by 2029, growing at a CAGR of 8.41% during the forecast period (2024-2029).
The impact of COVID-19 on the pharmaceutical analytical market was significant owing to the increase in demand for developing new drug candidates and vaccines to control the spread of the coronavirus disease. Since, analytical testing is required at all phases of drug development to monitor accuracy, efficiency, and safety. Due to the uncontrolled and sudden increase in covid-19 cases, the development of biosimilars, combination molecules, and other innovative vaccines and medicines has grown, which has resulted in an increased demand for significant bioanalytical testing such as electrophoresis, electrochemical and titrimetric assays, and immunoassays.
In addition, the increasing number of clinical trials, focus on analytical testing of biologics and biosimilars and increased trend of outsourcing laboratory testing services are actively affecting the growth of the studied market.
According to the FDA drug recall statistics, approximately 1,279 drugs are recalled every year globally, and 94% of FDA drug recalls have been in the United States, followed by 4% of FDA drug recalls in Canada in 2021. From 2012 to 2021, the FDA issued 12,028 drug recalls in the United States. This emerges the need to test the products and ensure public safety before they are marketed. Medicines are intended for a better outcome in humans and animals as the poor quality of medicines would affect the health of the patients and the funding systems. Therefore, analytical testing holds an important role in ensuring the safety and efficacy of the drugs.
An increase in R&D activities, collaborations, and strategic partnerships is expected to drive market growth. For instance, in November 2021, Alcami Corporation, Inc. signed a master laboratory services agreement with Novavax. With this agreement, Novavax secured full-time equivalent (FTE) resources to provide analytical testing support for its recombinant nanoparticle protein-based COVID-19 vaccine candidate with Matrix-M adjuvant. Such developments are estimated to propel market growth.
Therefore, owing to the aforementioned factors the studied market is anticipated to witness growth over the analysis period. However, the complex regulatory framework for maintaining laboratories and challenges in the development of proper analytical techniques are likely to impede market growth.
Pharmaceutical Analytical Testing Market Trends
Stability Testing Segment is Expected to Exhibit a Significant Market Growth Over the Forecast Period
Stability testing is done to evaluate the capability of the drug to retain its properties throughout its shelf-life. The stability studies of pharmaceutical products or drugs are one of the most important parameters for the development of new drugs and new formulations. The stability testing is done based on five parameters: chemical, physical, microbiological, therapeutic, and toxicity. The degradation with respect to any of these parameters can lead to health hazards.
The prediction of shelf-life is vital for the pharmaceutical product development of all the dosage forms. It is also utilized to determine the storage conditions and suggest label instructions. The stability testing of pharmaceutical products ensures the maintenance of product quality, safety, and efficacy throughout the shelf life which are considered a prerequisite for the acceptance and approval of any pharmaceutical products. And these studies are required to follow the guidelines of the world health Organization. According to Clinicaltrial.gov 2022 update, 433,207 studies have been registered till the current year, out of which 53% are registered outside the United States and 31% are registered in the United States. Hence, these factors are expected to drive the segment growth.
The strategic initiatives taken by the key players such as product launches, approvals, partnerships is also fueling the segment growth. For instance, in July 2021, LGM Pharma launched its analytical testing and stability services to pharmaceutical developers and manufacturers, including compounding pharmacies. Such developments are estimated to boost the segment growth during the forecast period.
North America Holds a Significant Share in the Market and Expected to do Same During The Forecast Period
North America is expected to be a dominant region in the Pharmaceutical Analytical Testing market owing to the increasing number of R&D activities and investments.
Approximately 53 novel drugs were approved in 2020 and 50 were approved in 2021 as per the data provided by the Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA). Among the total drugs approved worldwide in 2020 and 2021, most of them were approved in the United States before any other country in the world as per FDA. Also, as per clinical trials, 136,276 clinical trials has been registered in the United States up to the current year which contributes 31% of the total registered clinical trials. The high frequency of drug approvals in the region is estimated to boost the market growth in the region during the forecast period.
Furthermore, according to NIH, the President's Budget includes USD 12.1 billion for the FY2023 in mandatory resources to support pandemic preparedness, including research and development of vaccines, diagnostics, and therapeutics against high priority viral families, biosafety and biosecurity, as well as to increase laboratory capacity and clinical trial infrastructure in the United States. These increasing number of clinical trials, number of drug approvals, and major companies' involvement in increased R&D expenditures are expected to boost the demand for pharmaceutical analytical testing market growth in the North American region.
Additionally, the strategic initiatives taken by the key players such as product launches, approvals, partnerships is also fueling the segment growth. For instance, in December 2021, Pace Analytical Services, a division of Pace Science and Technology Company, acquired Special Pathogens Laboratory to strengthen its capabilities in the industry. Such developments are estimated to propel market growth in the region.
Pharmaceutical Analytical Testing Industry Overview
The pharmaceutical analytical testing market is fragmented in nature due to the presence of a number of companies globally. The studied market consists of several international and local companies that hold the majority of the market shares and are well known, including Laboratory Testing Inc., Eurofins Scientific, SGS SA, Toxikon Inc., Pace Analytical Services. Intertek Pharmaceutical Services, Boston Analytical, West Pharmaceutical Services Inc., and Steris.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Number of Clinical Trials
- 4.2.2 Focus on Analytical Testing of Biologics and Biosimilars
- 4.2.3 Increased Trend of Outsourcing Laboratory Testing Services
- 4.3 Market Restraints
- 4.3.1 Complex Regulatory Framework for Maintaining Laboratories
- 4.3.2 Challenges in the Development of Proper Analytical Techniques
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Service Type
- 5.1.1 Bioanalytical Testing
- 5.1.2 Method Development & Validation
- 5.1.3 Stability Testing
- 5.1.4 Drug Substances Testing
- 5.1.5 Other Service Types
- 5.2 Geography
- 5.2.1 North America
- 5.2.1.1 United States
- 5.2.1.2 Canada
- 5.2.1.3 Mexico
- 5.2.2 Europe
- 5.2.2.1 Germany
- 5.2.2.2 United Kingdom
- 5.2.2.3 France
- 5.2.2.4 Italy
- 5.2.2.5 Spain
- 5.2.2.6 Rest of Europe
- 5.2.3 Asia-Pacific
- 5.2.3.1 China
- 5.2.3.2 Japan
- 5.2.3.3 India
- 5.2.3.4 Australia
- 5.2.3.5 South Korea
- 5.2.3.6 Rest of Asia-Pacific
- 5.2.4 Middle East and Africa
- 5.2.4.1 GCC
- 5.2.4.2 South Africa
- 5.2.4.3 Rest of Middle East and Africa
- 5.2.5 South America
- 5.2.5.1 Brazil
- 5.2.5.2 Argentina
- 5.2.5.3 Rest of South America
- 5.2.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Laboratory Testing Inc.
- 6.1.2 Eurofins Scientific
- 6.1.3 SGS SA
- 6.1.4 Labcorp (Toxikon Inc)
- 6.1.5 Pace Analytical Services
- 6.1.6 Intertek Phamaceutical Services
- 6.1.7 Boston Analytical
- 6.1.8 West Pharmaceutical Services Inc.
- 6.1.9 Steris

















