
|
시장보고서
상품코드
1440279
태아 심박수 모니터링 기기 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Fetal Heart Rate Monitoring Device - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
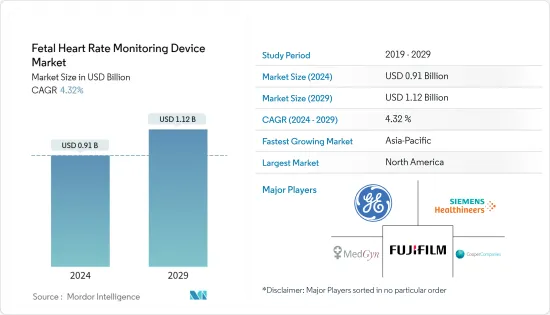
태아 심박수 모니터링 기기 시장 규모는 2024년 9억 1,000만 달러에 이를 것으로 추정됩니다. 2029년에는 11억 2,000만 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 4.32%의 연평균 복합 성장률(CAGR)을 나타낼 전망입니다.

코로나19의 출현으로 환자의 치료와 편안함에 대한 초점이 더욱 집중되고 있습니다. PLoS One이 2021년 7월에 발표한 연구에 따르면, 2020년 3월 코로나19의 첫 번째 물결이 시작될 때 선택적 수술의 발생률이 빠르게 감소했지만 5월과 6월에 회복되어 이후 수술 발생률이 참고 문헌보다 22% 더 높았다고 합니다. 응급 수술만 허용되고 신종 코로나 바이러스 감염에 영향을 받은 환자에게 자원을 전환하여 대부분의 심장 수술이 취소되거나 연기되면서 태아 심박수 모니터링 시장은 큰 타격을 입었습니다. 그러나 수요 증가로 인해 불가피한 상황이 발생하자 미국 식품의약국(FDA) 등 규제 당국은 어려운 상황에 처한 산모와 태아에게 중단 없는 지원과 치료를 제공하기 위해 의료기기 출시에 대한 규제를 어느 정도 완화했습니다. 따라서 코로나19는 팬데믹 이후에도 태아 심박수 모니터링 기기 시장에 긍정적인 영향을 미칠 것으로 예상됩니다.
조사 대상 시장의 성장은 주로 태아 모니터링 기기의 기술 발전, 출산율과 조산 증가, 더 나은 산모와 태아를 더 잘 돌보기 위한 정부 및 비정부 노력 증가에 기인합니다. 모니터링 기기의 기술 발전은 주로 환자 관리와 편안함에 초점을 맞추고 있기 때문에 시장 성장을 가속할 수 있습니다. 시장에 출시 된 무선 및 가정용 모니터링 기기는 의료 종사자와의 데이터 공유를 가능하게합니다. 조산은 임신 37주 이전에 아기가 태어나는 것을 말합니다. 이 일반적인 원인은 당뇨병이나 고혈압과 같은 생활습관입니다. 예를 들어, 국제 산부인과 산부인과 저널이 2020년 7월에 발표한 기사에 따르면 조산은 임신 37주 이전에 출산하는 것으로, 전 세계적으로 매년 약 1,500만 명의 아기가 조산으로 태어나고 있으며, 전 세계 조산율은 약 11%에 달할 전망입니다. 출산율은 약 11%입니다. 같은 소식통에 따르면 5세 미만의 조산으로 인해 100만 명의 어린이가 사망했으며, 조산은 어린이 사망의 주요 원인으로 전체 5세 미만 어린이 사망의 18%를 차지하며, 더 많은 어린이가 사망하고 있습니다. 전체 신생아 사망의 35%. 따라서 심박수를 정확하게 모니터링하는 것은 태아의 안전을 판단하는 데 도움이 될 수 있습니다. 따라서 조산 건수 증가와 태아 건강 모니터링에서 이러한 장치의 사용 증가로 인해 시장은 향후 상당한 성장률로 성장할 수 있습니다.
더 나은 시설을 확보하기 위해 다양한 정부 기관과 비정부 기관도 참여하고 있습니다. 예를 들어, WHO는 보건부와 협력하여 특히 출생시 및 생후 1주간의 치료를 강화하기 위해 투자했습니다. 이는 조직의 참여를 촉진하고 시장 성장을 가속할 수 있습니다. 앞서 언급 한 사실로 인해 태아 심박수 모니터링 기기 시장은 예측 기간 동안 상당한 성장을 이룰 것으로 예상됩니다.
심장 모니터링 기기의 주요 시장 기업의 다양한 발전, 제품 승인, 출시, 제휴 및 인수로 인해 예측 기간 동안 조사 대상 시장의 성장이 촉진될 수 있습니다. 2021년 6월, 미국 식품의약국(FDA)의 승인을 받고 특허를 보호받는 처방전 기반 원격 임신 모니터링 플랫폼인 Nuvo의 INVU를 상용화하는 민간 기업 Nuvo Group은 미국 식품의약국(FDA)으로부터 추가 허가를 받았다고 발표했습니다. 자궁 활동(UA) 원격 모니터링 기능을 제공하는 새로운 자궁 활동 모듈. 이러한 제품의 출시로 시장 성장이 기대됩니다.
그러나 의료기기에 대한 엄격한 규제 기준과 태아 심박수 모니터링 기기와 관련된 높은 비용은 조사 대상 시장 성장 억제요인으로 작용할 수 있습니다.
태아 심박수 모니터링 기기 시장 동향
내부 심박수 모니터링 기기 부문은 예측 기간 동안 성장할 것으로 예상
내부 태아 심박수 모니터링에는 태아의 피부에 직접 연결된 전자 변환기가 사용됩니다. 와이어 전극은 자궁 경부 구멍을 통해 태아의 두피 또는 기타 신체 부위에 부착되어 모니터에 연결됩니다. 이 유형의 전극은 때때로 나선형 전극 또는 두피 전극이라고도합니다.
기술적으로 진보된 많은 의료기기 조직들이 이러한 기기를 출시할 계획이거나 출시를 앞두고 있습니다. 이러한 무선 또는 케이블이 없는 모니터링 기기는 특히 산부인과 의사가 부족한 신흥 국가에서 산전 진료를 더 잘 할 수 있게 해줍니다. 또한, 블루투스를 통해 태아 심박수 데이터를 전송할 수 있는 무선 태아 모니터의 사용이 정상 분만 유도제를 투여받는 건강한 환자를 대상으로 시험적으로 시험되고 있으며, 집에서 24시간 모니터링할 수 있게 되었습니다. 가정에서 무선 태아 모니터링 기술을 사용하는 것은 임산부에게 실현 가능하고 받아들여질 수 있는 가능성이 있습니다. 이 기기는 H-cube(Her healthcare at home)의 HeraBEAT(데이터 공유 기능이 있는 스마트 태아 도플러)와 마찬가지로 임산부가 태아의 심박수를 듣고 모니터링하고 의료진과 공유할 수 있게 해줍니다. 따라서 더 나은 산전 관리를 제공하기 위한 기술적으로 진보된 솔루션의 지원으로 무선 태아 심박수 모니터링 기기에 대한 수요는 향후 증가할 것으로 예상됩니다.
예를 들어, 2022년 2월에 발표된 '신경아세포종 치료'라는 제목의 기사에 따르면, 신경아세포종의 유병률은 출생아 7,000명당 약 1명입니다. 15 세 미만 어린이의 발생률은 연간 백만 명당 10.54 명입니다. 같은 소식통에 따르면 환자의 약 37%가 영아로 진단을 받았고, 90%가 5세 미만으로 진단을 받았으며, 진단 시점의 평균 연령은 19개월입니다. 이러한 높은 질병 부담은 시장 성장을 주도하고 있습니다.
따라서 앞서 언급한 발전으로 인해 시장은 예측 기간 동안 상당한 성장을 이룰 것으로 예상됩니다.
북미는 예측 기간 동안 성장할 것으로 예상
북미는 미국과 캐나다와 마찬가지로 세계에서 가장 잘 구조화되고 발전된 선진 의료 시스템을 갖춘 국가 중 하나로 알려져 있습니다. 태아 및 신생아 치료 장비에 대한 연구개발비용이 높기 때문에 북미 정부도 헬스케어 연구를 위한 소규모 시설을 장려하고 있습니다. 따라서 많은 기업들이 이 지역에서 사업을 전개하도록 장려하여 북미가 전 세계 시장을 독점할 수 있도록 돕고 있습니다.
CDC의 2021년 11월 최신 정보에 따르면, 2020년 미국에서 태어난 영아 10명 중 1명이 조산의 영향을 받은 것으로 나타났습니다. 또한 아프리카계 미국인 여성의 조산율(14.4%)은 백인 또는 히스패닉 여성의 조산율(각각 9.1%와 9.8%)보다 약 50% 더 높다고보고했습니다. 조산아는 심장 합병증 위험이 높기 때문에 태아 심박수 모니터링 기기는 상태를 모니터링하는 데 도움이 되어 시장 성장을 가속할 수 있습니다.
또한, 미국 내 R&D 활동에 대한 정부 기관의 자금 지원 증가는 이 지역 시장 성장에 크게 기여하고 있습니다. 또한, 지속적인 기술 발전, 심장 모니터링에 대한 환자 인식 증가, 확립 된 의료 인프라 및 유리한 상환 정책으로 인해 북미의 심장 모니터링 시장이 확대될 것으로 예상됩니다. 예를 들어, 2021년 7월 애보트는 삽입형 심장 모니터(ICM) Jot Dx를 미국에서 출시했습니다. 이 기술은 환자의 부정맥을 원격으로 감지하고 진단 정확도를 향상시킬 수 있습니다.
따라서 앞서 언급한 요인으로 인해 조사 대상 시장은 북미에서 성장할 것으로 예상됩니다.
태아 심박수 모니터링 기기 산업 개요
태아 심박수 모니터링 기기 시장은 세계 및 지역의 여러 기업와 적당히 경쟁하고 있습니다. 시장 점유율 측면에서 볼 때 현재 주요 기업들이 시장을 독점하고 있습니다. 환자의 인식 수준이 높아지고 조산율이 높아짐에 따라 예측 기간 동안 많은 지역 기업이 태아 심박수 모니터링 기기 시장에 진입할 것으로 예상됩니다. 시장의 주요 기업은 GE Healthcare, Fujifilm Holdings Corporation, MedGyn Products Inc, Siemens Healthineers, Cooper Companies Inc, Edaninstruments, Trismed입니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 시장 성장 억제요인
- 업계의 매력 - Porter의 Five Forces 분석
- 바이어의 교섭력
- 공급 기업의 교섭력
- 신규 진출업체의 위협
- 대체 제품의 위협
- 경쟁 기업간 경쟁도
제5장 시장 세분화
- 제품 유형별
- 체내 태아 심박수 모니터링 기기
- 외부 태아 심박수 모니터링 기기
- 기술 유형별
- 도플러 초음파 장비
- 전자 태아 모니터링 장비
- 디바이스 휴대성별
- 휴대용
- 비휴대용
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 세계 기타 지역
- 북미
제6장 경쟁 구도
- 기업 개요
- Koninklijke Philips NV
- GE Healthcare
- Siemens Healthineers
- Cooper Companies Inc.
- MedGyn Products Inc.
- Edan Instruments
- Bionet America
- Trismed Co. Ltd
- Mindray Medical International Limited
- Fujifilm Holdings Corporation
- Natus Medical Inc.
제7장 시장 기회와 향후 동향
LSH 24.03.14The Fetal Heart Rate Monitoring Device Market size is estimated at USD 0.91 billion in 2024, and is expected to reach USD 1.12 billion by 2029, growing at a CAGR of 4.32% during the forecast period (2024-2029).
With the emergence of COVID-19, the focus has shifted more toward patient care and comfort. The study published in July 2021 by PLoS One stated that the elective procedure incidence decreased rapidly at the onset of the first COVID-19 wave in March 2020 but recovered in May and June, after which the surgery incidence was 22% higher than in the reference years. With only urgent surgeries being allowed and most of the cardiac procedures canceled or postponed due to the diversion of resources toward COVID-19-affected patients, the market for fetal heart rate monitoring market was tremendously affected. However, with the rise in demand compelling the situation, regulatory authorities like the United States Food and Drug Administration provided some relaxation in the regulations for the launch of medical devices to provide uninterrupted support and care to the mother and fetus during the challenging situation of the pandemic. Thus, COVID-19 is expected to impact the fetal heart rate monitoring device market positively over the post-pandemic era.
The growth of the market studied is largely attributed to technological advancement in fetal monitoring devices, increasing birth rate and preterm births, and rising government and non-government initiatives to provide better maternal and fetal care. The technological advancement in monitoring devices is likely to drive the market's growth, as it primarily focuses on patient care and comfort. The wireless and in-home monitoring devices introduced in the market enable data sharing with healthcare workers. Preterm birth indicates that a baby is born before the completion of 37 weeks of pregnancy, and lifestyle conditions, such as diabetes and hypertension, are the common causes for this. For instance, as per the article published in July 2020 by the International Journal of Gynecology & Obstetrics, preterm birth is a live birth that occurs before 37 completed weeks of pregnancy, and approximately 15 million babies are born preterm annually worldwide, indicating a global preterm birth rate of about 11%. As per the same source, with 1 million children dying due to preterm birth before the age of 5 years, preterm birth is the leading cause of death among children, accounting for 18% of all deaths among children aged under five years and as many as 35% of all deaths among newborns. Therefore, vigorous heart rate monitoring helps determine the fetus's safety. Hence, owing to the rising number of preterm births and rising usage of these devices in fetal health monitoring, the market is likely to grow with a notable growth rate over the upcoming period.
Various government and non-government agencies also participate to ensure better facilities. For instance, WHO collaborated with ministries of health to strengthen and invest in care, particularly around the time of birth and the first week of life. This is likely to encourage the participation of the organization and boost the market's growth. Due to the aforementioned facts, the fetal heart rate monitoring devices market is expected to witness significant growth over the forecast period.
Various advancements, product approvals, launches, partnerships, and acquisitions by the key market players in cardiac monitoring devices are likely to boost the growth of the market studied over the forecast period. In June 2021, Nuvo Group-a private company commercializing INVU by Nuvo, an FDA-cleared, patent-protected, prescription-initiated remote pregnancy monitoring platform, announced that it had received clearance from the US Food and Drug Administration (FDA) to add a new uterine activity module that provides the capability for remote monitoring of uterine activity (UA). Such product launches are anticipated to result in the growth of the market.
However, stringent regulatory norms for medical devices and the high cost associated with fetal heart rate monitoring devices may act as restraining factors for the market studied.
Fetal Heart Rate Monitoring Devices Market Trends
Internal Heart Rate Monitoring Devices Segment is Expected to Witness Growth over the Forecast Period
Internal fetal heart rate monitoring uses an electronic transducer connected directly to the fetal skin. A wire electrode is attached to the fetal scalp or another body part through the cervical opening and is connected to the monitor. This type of electrode is sometimes called a spiral or scalp electrode.
Many technologically advanced medical device organizations are planning to launch such devices or are on the verge of launching them. These wireless or cableless monitoring devices have made prenatal care a better experience, especially in developing countries with a shortage of obstetricians and gynecologists. Additionally, the use of a wireless fetal monitor that can transmit fetal heart rate data via Bluetooth was piloted among healthy patients undergoing labor induction at term and allowed home monitoring for 24 hours. The use of wireless fetal monitoring technology in the home may be found feasible and acceptable to pregnant women. The instrument, like HeraBEAT (smart fetal doppler with data sharing) by H-cube (Her Healthcare at home), enables the expecting mother to hear and monitor the fetus's heart rate and share it with health practitioners. Thus, with the support of technologically advanced solutions for providing better prenatal care, the demand for wireless fetal heart rate monitoring devices is expected to grow in the future.
For instance, according to the article titled 'Neuroblastoma Treatment' published in February 2022, the prevalence of neuroblastoma is about 1 case per 7,000 live births. The incidence is 10.54 cases per 1 million annually in children younger than 15 years. As per the same source, about 37% of patients are diagnosed as infants, and 90% are younger than 5 years at diagnosis, with a median age at diagnosis of 19 months. Such a high burden of disease is propelling the growth of the market.
Thus, due to the aforementioned developments, the market is expected to witness significant growth over the forecast period.
North America is Expected to Witness Growth over the Forecast Period
North America, along with the United States and Canada, is found to have one of the most well-structured, developed, and advanced healthcare systems in the world. With high R&D expenditure for fetal and neonatal care devices, governments of the North American region are also promoting small setups for healthcare research. Therefore, many companies are encouraged to operate in the region, allowing North America to dominate the market across the world.
According to the November 2021 update by the CDC, in 2020, preterm birth affected 1 in every 10 infants born in the United States. It also reported that the rate of preterm birth among African-American women (14.4%) was about 50% higher than the rate of preterm birth among white or Hispanic women (9.1% and 9.8%, respectively). Since infants who are born preterm are at an increased risk of heart complications, fetal heart rate monitoring device helps in the monitoring of the conditions, thereby driving the growth of the market.
Moreover, the increasing funding from government organizations for research and development activities in the United States is a major contributing factor to the market's growth in this region. Furthermore, continuous advancements in technology, growing awareness in patients about cardiac monitoring, well-established healthcare infrastructure, and favorable reimbursement policies are expected to boost the cardiac monitoring market in the North American region. For instance, in July 2021 Abbott launched Jot Dx, the insertable cardiac monitor (ICM), in the United States. This technology allows for remote detection and improved diagnostic accuracy of cardiac arrhythmias in patients.
Therefore, owing to the aforementioned factors, the growth of the market studied is anticipated in the North American region.
Fetal Heart Rate Monitoring Devices Industry Overview
The fetal heart rate monitoring devices market is moderately competitive with several global and regional players. In terms of market share, the major players currently dominate the market. With the rising patient awareness levels and high preterm birth rates, many regional players are expected to be part of the fetal heart rate monitoring devices market over the forecast period. The market's major players are GE Healthcare, Fujifilm Holdings Corporation, MedGyn Products Inc., Siemens Healthineers, Cooper Companies Inc, Edan instruments, and Trismed Co. Ltd.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Technological Advancement in Fetal Monitoring Devices
- 4.2.2 Rising Number of Birth Rate and Preterm Births
- 4.2.3 Rising Government and Non-government Initiatives to Provide Better Maternal and Fetal Care
- 4.3 Market Restraints
- 4.3.1 Strigent Regulatory Norms for Medical Devices
- 4.3.2 High Cost Associated with Fetal Heart Rate Monitoring Devices
- 4.4 Industry Attractiveness - Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Buyers/Consumers
- 4.4.2 Bargaining Power of Suppliers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Product Type
- 5.1.1 Internal Fetal Heart Rate Monitoring Device
- 5.1.2 External Fetal Heart Rate Monitoring Device
- 5.2 By Technology Type
- 5.2.1 Doppler Ultrasound Device
- 5.2.2 Electronic Fetal Monitoring Device
- 5.3 By Portablity of Device
- 5.3.1 Portable
- 5.3.2 Non-portable
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Rest of the World
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Koninklijke Philips NV
- 6.1.2 GE Healthcare
- 6.1.3 Siemens Healthineers
- 6.1.4 Cooper Companies Inc.
- 6.1.5 MedGyn Products Inc.
- 6.1.6 Edan Instruments
- 6.1.7 Bionet America
- 6.1.8 Trismed Co. Ltd
- 6.1.9 Mindray Medical International Limited
- 6.1.10 Fujifilm Holdings Corporation
- 6.1.11 Natus Medical Inc.















