
|
시장보고서
상품코드
1440281
웨어러블 제세동기 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Wearable Cardioverter Defibrillators - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
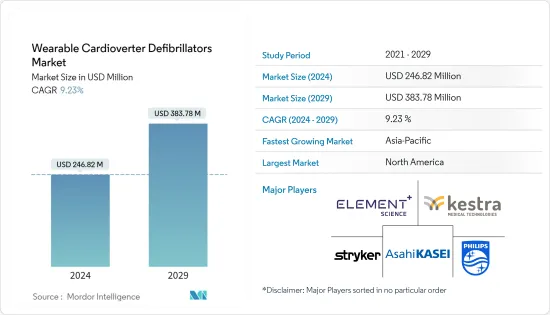
웨어러블 제세동기 시장 규모는 2024년 2억 4,682만 달러에 이를 것으로 추정됩니다. 2029년에는 3억 8,378만 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 9.23%의 연평균 복합 성장률(CAGR)을 나타낼 전망입니다.

COVID19 팬데믹은 웨어러블 제세동기 시장 성장에 큰 영향을 미쳤습니다. 2022년 2월Nature Medicine에 게재 된 기사에 따르면 COVID-19 감염 환자는 심부전, 혈전 색전증, 부정맥, 허혈성 및 비 허혈성 심장 질환, 심낭염, 심근염, 허혈성 및 비 허혈성 심장 질환, 허혈성 및 비 허혈성 심장 질환과 같은 심혈관 질환에 걸릴 가능성이 더 높다는 사실이 관찰되었습니다. -허혈성 심장병. 따라서 CVD 관련 질환의 높은 부담으로 인해 임상의의 비접촉식 통신 및 병력 추적이 가능하기 때문에 대유행 기간 동안 심장 박동 리듬을 모니터링하기 위해 웨어러블 제세동기 장치에 대한 수요가 증가했습니다. 또한, 제한이 해제되고 심장 치료 및 서비스가 재개됨에 따라 예측 기간 동안 시장 성장이 예상됩니다.
심혈관 질환의 유병률 증가, 노인 인구 증가, 비침습적 장치의 사용 편의성 등의 요인이 시장 성장을 가속하고 있습니다. 예를 들어, BHF의 2022년 보고서에 따르면 2021년 영국에서 760만 명 이상이 심혈관 질환을 앓고 있는 것으로 나타났습니다. 따라서 심혈관 질환과 높은 유병률로 인해 심장 박동 리듬의 정기적 인 모니터링에 대한 수요가 증가하여 시장을 주도할 것으로 예상됩니다. 또한, 2022년 10월 CDC의 최신 정보에 따르면, 미국에서는 매년 약 80만 5,000명이 심장마비로 사망하고 있습니다. 따라서 심혈관 질환과 그 유병률이 높기 때문에 심장 박동 리듬의 정기적 인 모니터링에 대한 수요가 증가하여 시장 성장을 가속할 것으로 예상됩니다.
또한, 인구의 비만, 당뇨병, 고혈압, 고콜레스테롤 유병률 증가는 시장 성장에 기여하고 있습니다. 유니세프 세계 비만 아틀라스가 발표한 2022년 통계에 따르면, 인도에서는 2030년까지 2,700만 명 이상의 어린이가 비만으로 고통받을 것으로 예상됩니다. 따라서 예상되는 비만 인구 증가로 인해 혈류가 감소하여 심장 마비를 유발하는 심방 세동(AF)의 위험이 증가 할 수 있습니다. 이는 제세동기에 대한 수요를 촉진하고 시장 성장을 가속할 것으로 예상됩니다.
또한, IDF가 발표한 2022년 통계에 따르면, 전 세계적으로 20세에서 79세 사이의 성인 약 5억 3,700만 명이 당뇨병을 앓고 있습니다. 이 숫자는 2030년까지 6억 4,300만 명, 2045년까지 7억 8,300만 명으로 증가할 것으로 예측됩니다. 당뇨병으로 인한 고혈당은 심장과 혈관을 조절하는 신경을 손상시켜 관상 동맥 질환과 같은 다양한 심혈관 질환을 유발할 수 있으며, 심장의 전기 전도 시스템에 영향을 미쳐 심방 세동과 심실 부정맥을 유발할 수 있습니다. 이로 인해 일반적인 심장 상태 및 조율 모니터링의 필요성이 증가하여 시장 성장을 더욱 촉진할 것으로 예상됩니다.
또한, 제품 승인 증가와 기술적으로 진보된 제품 개발이 증가하면서 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, 2021년 8월 미국 식품의약국(FDA)은 센서, 심장 리듬 모니터, 소형 자동 체외 제세동기를 갖춘 웨어러블 기기인 Kestra Medical Technologies ASSURE 웨어러블 제세동기(WCD) 시스템에 대해 시판 전 승인을 부여했습니다. 이 제품은 심장 돌연사 위험이 있는 환자를 모니터링하고 치료하는 것을 목적으로 합니다.
따라서, 심혈관 질환 및 비만 부담 증가와 같은 요인과 제품 출시는 예측 기간 동안 시장 성장에 기여할 것으로 예상됩니다. 그러나 웨어러블 기기의 규제 불확실성과 프라이버시 및 정보 보안 문제는 예측 기간 동안 시장 성장을 저해할 것으로 예상됩니다.
웨어러블 제세동기 시장 동향
성인 부문은 조사 대상 시장에서 높은 CAGR을 기록할 것으로 예상
성인 부문은 예측 기간 동안 상당한 성장을 보일 것으로 예상됩니다. 시장 성장에 기여하는 요인은 비정상적인 심장 박동 리듬 및 부정맥과 같은 심혈관 질환을 앓고 있는 환자의 발생률 증가와 성인에서 이러한 질환의 유병률 증가입니다.
Heart Rhythm이 2021년 12월에 발표한 기사에 따르면, 선천성 심장 질환을 가진 성인은 심부전 및 부정맥을 자주 경험할 수 있는 것으로 관찰되었습니다. 같은 소식통에 따르면 심부전(HF)은 성인 CHD 환자, 특히 40세 이상 환자에서 매우 널리 퍼져 있으며 가장 흔한 사망 원인입니다. 따라서 다른 심장 질환을 앓고 있는 성인은 빈맥 및 서맥성 부정맥이 발생할 위험이 높기 때문에 첨단 제세동기에 대한 수요가 증가할 것으로 예상됩니다. 이는 이 부문의 성장을 가속할 것으로 예상됩니다.
또한 호주 통계청의 2022년 3월 최신 정보에 따르면 2020-2021년 호주의 심장병 유병률은 4.0%로, 이는 약 100만 명에 해당합니다. 이 소식통에 따르면 호주에서 심장병은 연령에 따라 증가하여 45-54세 2.3%에서 75세 이상 23.2%로 증가했으며, 호주에서 가장 많은 심장병을 앓고 있는 사람은 남성이라고 합니다. 따라서 CVD 부담 증가와 노인 인구 증가는 예측 기간 동안 조사 대상 부문의 성장을 가속하는 주요 요인이 될 것으로 예상됩니다.
또한, 심장 박동 문제를 치료하기 위한 기술적으로 진보된 웨어러블 제세동기 개발에 주력하는 스타트업들이 이 부문의 성장에 기여하고 있습니다. 예를 들어, 2022년 4월 Kestra Medical Technologies는 자사의 ASSURE 웨어러블 제세동기(WCD) 시스템이 갑작스런 심장 마비 위험에 처한 환자를 보호하는 차세대 모니터링 및 치료법으로 입증되었습니다고 보고했습니다.
따라서, 고령 인구 증가와 기업 활동의 활성화에 따른 심혈관계 질환 부담 증가 등의 요인으로 인해 예측 기간 동안 조사 대상 부문이 증가할 것으로 예상됩니다.
북미가 시장을 주도하고 있으며, 예측 기간 동안에도 비슷한 성장세를 보일 것으로 예상
예측 기간 동안 북미가 시장을 주도할 것으로 예상됩니다. 시장 성장에 기여하는 요인으로는 고령화 인구 증가에 따른 심혈관계 부담 증가, 고가의 의료 서비스 및 상환 정책, 지역 내 비침습적 기기 채택 증가 등이 있습니다.
심혈관 질환의 유병률 증가는 제세동기 장치에 대한 수요를 촉진하는 중요한 요인입니다. 미국심장협회(AHA)가 발표한 통계에 따르면 2035년까지 미국 성인 인구의 약 45%가 심혈관 질환을 앓을 것으로 예상됩니다. 또한 CDC가 발표한 2022년 통계에 따르면 약 1,210만 명이 심혈관 질환에 걸릴 것으로 예상됩니다. 미국에서는 2030년까지 심방세동으로 인한 사망자 수가 약 1억 명에 달할 것으로 예상되며, 2021년 6월 CIHI가 발표한 자료에 따르면 캐나다에서는 연간 약 6만 2000건의 뇌졸중이 보고되고 있으며, 뇌졸중은 사망 원인 중 3위를 차지하고 있습니다. 또한 2021년 7월 Cardiovascular and Metabolic Science에 게재된 논문에 따르면, 멕시코에서는 40세 이상에서 허혈성 심장질환이 많이 발생하는 것으로 관찰됐습니다. 따라서 심혈관 질환을 앓고 있는 사람들의 수가 증가할 것으로 예상되며, 심방세동 및 부정맥의 위험이 증가하여 심장마비를 더욱 예방하기 위해 정기적인 심박수 모니터링이 필요할 것으로 예상됩니다. 이는 예측 기간 동안 제세동기에 대한 수요를 증가시켜 시장 성장을 가속할 것으로 예상됩니다.
또한, 국내 노인 인구 증가는 두꺼운 동맥 경화 증가로 인해 심혈관 질환이 발생하기 쉬워 고혈압 및 기타 심장 박동 관련 문제를 유발할 가능성이 높습니다. 이로 인해 정기적으로 심장 리듬을 모니터링하는 웨어러블 제세동기에 대한 수요도 증가하여 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, UNPF가 발표한 2022년 통계에 따르면 2022년 미국 인구의 약 17%가 65세 이상이 될 것으로 예상됩니다. 이 소식통에 따르면 2022년에는 캐나다 인구의 약 19%, 멕시코 인구의 8%가 65세 이상이 될 것이라고 합니다.
따라서 이 지역의 노인 인구 증가와 심방세동 및 기타 심혈관 질환의 유병률 증가와 같은 앞서 언급한 요인으로 인해 조사 대상 시장은 예측 기간 동안 증가할 것으로 예상됩니다.
웨어러블 세동제세동기 산업 개요
웨어러블 제세동기 시장은 적당히 통합되어 있으며, 전 세계적으로 기업이 거의 없습니다. 시장 점유율 측면에서 현재 시장을 독점하고 있는 주요 업체는 거의 없습니다. 환자의 인식 수준이 높아지고 질병 유병률이 높아짐에 따라 예측 기간 동안 많은 지역 기업이 웨어러블 제세동기 시장에 참여할 것으로 예상됩니다. 시장 주요 기업으로는 LivaNova PLC, Koninklijke Philips NV, Zoll Medical Corporation, Stryker Corporation, Medtronic PLC, Nihon Koden Corporation, Kestra Medical Technologies Inc., Boston Scientific Corporation, Element Science 등이 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 시장 성장 억제요인
- 업계의 매력 - Porter의 Five Forces 분석
- 신규 진출업체의 위협
- 바이어의 교섭력
- 공급 기업의 교섭력
- 대체 제품의 위협
- 경쟁 기업간 경쟁도
제5장 시장 세분화
- 인구별
- 소아
- 성인
- 고령자
- 최종사용자별
- 가정
- 병원 및 심장병 클리닉
- 기타 최종사용자
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 세계 기타 지역
- 북미
제6장 경쟁 구도
- 기업 개요
- LivaNova PLC
- Koninklijke Philips NV
- Asahi Kasei Corporation(ZOLL Medical Corporation)
- Stryker Corporation
- Medtronic PLC
- Nihon Kohden Corporation
- Kestra Medical Technologies Inc.
- Boston Scientific Corporation
- Element Science Inc.
제7장 시장 기회와 향후 동향
LSH 24.03.14The Wearable Cardioverter Defibrillators Market size is estimated at USD 246.82 million in 2024, and is expected to reach USD 383.78 million by 2029, growing at a CAGR of 9.23% during the forecast period (2024-2029).
The COVID-19 pandemic significantly impacted the growth of the wearable cardioverter defibrillator market. An article published in Nature Medicine in February 2022 observed that people with COVID-19 are more likely to have cardiovascular diseases, such as heart failure, thromboembolic disorders, dysrhythmias, ischemic and non-ischemic heart disease, pericarditis, myocarditis, and ischemic and non-ischemic heart disease. Thus, the high burden of CVD-related diseases raised the demand for wearable cardioverter defibrillator devices to monitor heart rhythm during the pandemic period as they allowed contactless communication and tracking of medical conditions by clinicians. Moreover, with released restrictions and resumed cardiac treatment and services, the studied market is expected to grow over the forecast period.
Factors such as the increasing prevalence of cardiovascular disorders, the rising geriatric population, and the ease of use of non-invasive devices are boosting the market growth. For instance, as per BHF's 2022 report, more than 7.6 million people in the United Kingdom were living with cardiovascular diseases in 2021. Hence, cardiovascular diseases and their high prevalence is expected to increase the demand for regular monitoring of heart rhythm, propelling the market growth. Also, according to the October 2022 update of the CDC, about 805,000 people in the United States have a heart attack every year. Hence, cardiovascular diseases and their high prevalence is expected to increase the demand for regular monitoring of heart rhythm, propelling the market growth.
In addition, the increasing prevalence of obesity, diabetes, hypertension, and high cholesterol among the population is contributing to market growth. According to the 2022 statistics published by UNICEF World Obesity Atlas, more than 27 million children will suffer from obesity by 2030 in India. Thus, the expected increase in the obese population may increase the risk of atrial fibrillation (AF), which reduces blood flow and leads to a heart attack. This is anticipated to propel the demand for cardioverter defibrillator devices, bolstering the market growth.
Also, according to the 2022 statistics published by IDF, about 537 million adults aged between 20 and 79 were living with diabetes globally. This number is projected to increase to 643 million and 783 million by 2030 and 2045, respectively. High blood sugar caused by diabetes can damage the nerves that control the heart and blood vessels, leading to a variety of cardiovascular diseases, such as coronary artery disease, that impact the electrical conduction system in the heart, resulting in atrial fibrillation and ventricular arrhythmias. This raises the need for a common heart condition and rhythm monitoring, further expected to augment market growth.
Furthermore, the rising product approvals and increasing development of technologically advanced products are also expected to increase the market growth. For instance, in August 2021, the United States Food and Drug Administration granted pre-market approval to Kestra Medical Technologies ASSURE wearable cardioverter defibrillator (WCD) system, a wearable device with incorporated sensors, a cardiac rhythm monitor and a miniaturized automated external defibrillator. It is intended for the monitoring and treatment of patients who are at risk of sudden cardiac death.
Therefore, owing to the factors such as the growing burden of cardiovascular diseases and obesity, coupled with the launch of products, are expected to contribute to the growth of the market over the forecast period. However, regulatory uncertainty and privacy and information security issues in wearable devices are expected to hinder market growth over the forecast period.
Wearable Cardioverter Defibrillators Market Trends
Adult Segment Expected to Register a High CAGR in the Studied Market
The adult segment is expected to witness significant growth over the forecast period. The factors attributing to the market growth are the increasing incidence of patients suffering from cardiovascular disorders, such as abnormal heart rhythms or arrhythmias, and an increase in the prevalence of these disorders in adults.
According to an article published by Heart Rhythm in December 2021, it has been observed that adults with congenital heart disease may frequently experience heart failure and arrhythmias. As per the same source, heart failure (HF) is highly prevalent in adults with CHD, particularly those over the age of 40 years, and is the most common cause of mortality. Thus, the high risk of developing tachyarrhythmias and bradyarrhythmias among the adult population suffering from other heart problems is expected to raise the demand for advanced cardioverter defibrillator devices. This is anticipated to fuel the segment's growth.
Furthermore, according to the March 2022 update of the Australian Bureau of Statistics, the prevalence of heart disease in Australia was 4.0% in 2020-2021, which equates to about 1 million people. According to the same source, heart disease increased with age in Australia, from 2.3% of people aged 45-54 years to 23.2% of people aged 75 years and over, with males being the most affected by it in the country. Hence, the rising burden of CVDs and the increasing geriatric population is expected to be the major driving factor for the growth of the studied segment over the forecast period.
Moreover, the rising company's focus on developing technologically advanced wearable cardioverter defibrillator devices to treat cardiac rhythm problems contributes to segment growth. For instance, in April 2022, Kestra Medical Technologies reported that its ASSURE Wearable Cardioverter Defibrillator (WCD) system proves to be the next generation of monitoring and therapy to protect patients at risk of sudden cardiac arrest.
Therefore, the studied segment is expected to increase over the forecast period due to factors such as the increasing burden of cardiovascular diseases coupled with the growing geriatric population and rising company activities.
North America Dominates the Market and Expects to do Same Over the Forecast Period
North America is expected to dominate the market over the forecast period. The factors attributing to the market growth are the rising cardiovascular burden coupled with the growing aging population, high healthcare expenditure and reimbursement policies, and growing adoption of non-invasive devices in the region.
The increasing prevalence of cardiovascular diseases is the key factor driving the demand for cardioverter defibrillator devices. According to the statistics published by the American Heart Association (AHA), about 45% of the adult population in the United States is expected to suffer from cardiovascular disease by 2035. Also, according to the 2022 statistics published by CDC, about 12.1 million people are expected to suffer from atrial fibrillation in the United States by 2030. As per the data posted by the CIHI in June 2021, about 62,000 strokes are reported annually in Canada, making it the third leading cause of death. Furthermore, per an article published in the Cardiovascular and Metabolic Science in July 2021, it has been observed that ischemic heart disease is high in people aged 40 years and above in Mexico. Thus, the expected increase in the number of people with cardiovascular diseases increases the risk of atrial fibrillation and cardiac arrhythmias, which requires regular heart rate monitoring to prevent a heart attack further. This is anticipated to fuel the demand for cardioverter defibrillators over the forecast period, propelling the market growth.
Furthermore, the rising geriatric population in the country is more prone to develop cardiovascular diseases due to increased stiffness of large arteries, which causes hypertension and other heart rhythm-related problems. This is also expected to increase the demand for wearable cardioverter defibrillator devices to monitor cardiac rhythm regularly, bolstering the market growth. For instance, according to the 2022 statistics published by UNPF, about 17% of the population will be 65 years and above in 2022 in the United States. According to the same source, about 19% of the people in Canada and 8% of the population in Mexico will be 65 years or above in 2022.
Therefore, the studied market is expected to increase over the forecast period due to the aforementioned factors, such as the rising prevalence of atrial fibrillation and other cardiovascular diseases, along with the growing geriatric population in the region.
Wearable Cardioverter Defibrillators Industry Overview
The wearable cardioverter defibrillator market is moderately consolidated, with few players globally. In terms of market share, few of the major players currently dominate the market. With the rising patient awareness levels and high prevalence of diseases, many regional players are expected to be part of the wearable cardioverter defibrillator market over the forecast period. Some of the major players in the market are LivaNova PLC, Koninklijke Philips NV, Zoll Medical Corporation, Stryker Corporation, Medtronic PLC, Nihon Kohden Corporation, Kestra Medical Technologies Inc., Boston Scientific Corporation, Element Science and others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope Of The Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevelance of Cardiovascular Disorders
- 4.2.2 Increasing Elderly Population
- 4.2.3 Ease of Use for Non-invasive Devices
- 4.3 Market Restraints
- 4.3.1 Regulatory Uncertainty
- 4.3.2 Privacy and Information Security Issues in Wearable Devices
- 4.4 Industry Attractiveness - Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD Million)
- 5.1 By Demography
- 5.1.1 Pediatric
- 5.1.2 Adults
- 5.1.3 Geriatric
- 5.2 By End-user
- 5.2.1 Home
- 5.2.2 Hospitals and Cardiology Clinics
- 5.2.3 Others End-users
- 5.3 By Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Rest of the World
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 LivaNova PLC
- 6.1.2 Koninklijke Philips NV
- 6.1.3 Asahi Kasei Corporation (ZOLL Medical Corporation)
- 6.1.4 Stryker Corporation
- 6.1.5 Medtronic PLC
- 6.1.6 Nihon Kohden Corporation
- 6.1.7 Kestra Medical Technologies Inc.
- 6.1.8 Boston Scientific Corporation
- 6.1.9 Element Science Inc.









