
|
시장보고서
상품코드
1849942
내시경 기기 : 시장 점유율 분석, 산업 동향, 통계, 성장 예측(2025-2030년)Endoscopy Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
내시경 기기 시장은 2025년에 401억 달러로 평가되었고, 2030년에 약 550억 9,000만 달러에 이를 것으로 예상되며, CAGR은 6.56%를 나타낼 전망입니다.

최소 침습 수술의 확산은 병원 자본 예산을 지속적으로 재편하며, 환자의 회복 기간을 단축하고 수술실 공간을 고수익 수술에 할당할 수 있게 하는 시각화 타워 및 첨단 영상 모듈에 투자를 유도하고 있습니다. 동시에 감염 관리 의무화로 인해 일회용 또는 부분 일회용 내시경으로 조달 방향이 전환되고 있으며, 이는 재처리 노동력을 줄일 뿐만 아니라 병원 감염 관련 보상 감액도 제한합니다. 아시아태평양 지역의 성장은 보험 적용 범위 확대와 숙련된 내시경 전문의 부족이 맞물리면서 글로벌 평균을 앞지르고 있으며, 이로 인해 해당 지역 구매자들은 학습 곡선을 단축시키는 직관적인 소프트웨어 기반 플랫폼을 선호하게 되었습니다. 북미의 기존 공급업체들은 여전히 핵심 고객이지만, 일회용 십이지장내시경의 빠른 채택으로 인해 제조사들은 프리미엄 가격과 물량 기반 비용 효율성 사이의 균형을 맞추도록 압박받고 있습니다.
세계의 내시경 기기 시장 동향 및 인사이트
외과 전문 분야 전반에 걸친 최소 침습 시술로의 광범위한 전환
전문 분야를 막론하고 임상의들은 최소 침습적 중재를 중심으로 치료 경로를 재구성하고 있습니다. 자연 구멍을 통한 내시경적 로봇 수술은 제품 로드맵이 동일한 가치 제안(환자 외상 감소, 입원 기간 단축, 빠른 회복)으로 수렴하는 방식을 보여줍니다. 두 번째 차원의 함의는 병원들이 이제 영상실 및 수술실 리모델링에 대한 자본 예산 요청을 이러한 기술로 인한 처리량 증가 주장과 연계한다는 점입니다. 따라서 내시경 공급업체들은 이미지 품질뿐만 아니라 측정 가능한 워크플로우 효율성 측면에서도 점점 더 경쟁해야 합니다.
내시경 검사의 시각화에서 끊임없는 혁신이 임상 성과를 향상
고해상도 광학 기술, 3D 영상, 소프트웨어 강화 대비 플랫폼은 초기 병변 검출을 개선했습니다. 예를 들어 올림푸스의 EVIS EXERA III는 좁은 대역 영상(NBI)과 듀얼 포커스 기술을 활용해 대장내시경 검사 중 선종 발견률을 높입니다. 의료 제공자에게 이는 더 작은 조직 부피를 선택적으로 생검할 수 있는 신기술로 이어지며, 이는 병리 검사 결과를 신속하게 제공할 뿐만 아니라 대규모로 소모품 비용을 절감합니다. 이러한 최적화는 외래 시설이 품질 기준을 충족하면서도 경쟁력 있는 보험 급여율을 유지할 수 있게 합니다.
훈련된 내시경 전문의 및 지원 인력의 글로벌 부족
적응증 확대 속도가 숙련된 내시경 전문의의 글로벌 공급량을 앞질렀습니다. 미국 위장관 내시경 학회(ASGE)는 ERCP 및 EUS와 같은 고급 시술이 장기간의 멘토링을 필요로 하여 신속한 자격 인증을 저해한다고 강조합니다. 공급업체에게 실용적인 결과는 통합 가이드 소프트웨어, 표준 치료 체크리스트, 인공지능 지원 내비게이션을 갖춘 시스템에 대한 신흥 시장이 등장하고 있다는 점입니다. 훈련 기간을 단축하는 기기는 활용률을 개선하여, 경쟁적인 자본 요청 사이에서 결정하는 관리자들의 비즈니스 사례를 강화합니다.
부문 분석
위장 내시경은 2024년 시장 점유율 54.9%를 차지하며 대장 내시경 및 상부 위장관 진단에 대한 높은 의존도를 반영했습니다. 그러나 비만 수술 재수술 및 자궁내막증 치료와 같은 적응증 확대로 복강경 검사는 2030년까지 연평균 8.9% 성장할 것으로 전망됩니다. 이러한 추세는 다학제적 수술실 팀이 복강경 및 내시경 모드 전환이 가능한 모듈식 타워 구성을 추진할 수 있음을 시사하며, 이는 고량 위장관 수술실과 유연한 다학제 수술실 간 조달 전략을 효과적으로 분할할 수 있습니다.
내시경 초음파(EUS) 역시 미국에서 증가하는 상부 위장관 EUS 시술 건수로 입증되듯 주목받고 있습니다. EUS가 병기 결정과 췌장신경절 신경절제술 같은 치료적 중재를 모두 용이하게 하기 때문에, 병원들은 이 기술을 진단 비용 센터가 아닌 수익 증대 보조 수단으로 포지셔닝하기 시작했으며, 이로 인해 예산 권한이 방사선과에서 위장관과로 미묘하게 이동하고 있습니다.
재사용 내시경은 2024년 기준 82% 점유율로 여전히 우세하지만, 일회용 제품은 연간 12.5% 성장 중입니다. 일회용 내시경과 표준 장치를 비교한 연구에 따르면 재처리 시간이 약 48분에서 10분 미만으로 단축되어 일일 처리량이 실질적으로 3배 증가합니다. 대부분의 외래수술센터(ASC)가 빡빡한 일일 일정으로 운영된다는 점을 고려하면 이 처리량 증가는 매우 매력적입니다. 진료 시간을 연장하지 않고도 룸당 추가 2-3건의 시술이 가능해지면 EBITDA 마진을 실질적으로 개선할 수 있습니다.
이러한 파급 효과로 시설 관리자들이 감염 위험 완화와 환경적 영향을 동시에 고려함에 따라 폐기물 관리 및 지속가능성에 대한 관심이 높아지고 있습니다. 이러한 이중적 고려사항은 향후 구매 기준을 재활용 가능 소재 및 회수 프로그램으로 전환시키는 데 영향을 미칠 것이며, 이는 제조사들에게 새로운 서비스 라인 수익 모델을 도입할 기회를 제공할 것입니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 지원
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 상황
- 시장 개요
- 시장 성장 촉진요인
- 위장관 및 대장암의 전 세계적 발병률 증가로 인한 검진 수요 촉진
- 외과 전문 분야 전반에 걸친 최소 침습적 시술로의 광범위한 전환
- 내시경 영상 기술의 지속적인 혁신으로 인한 임상 결과 개선
- 예방적 내시경을 지원하는 유리한 보험급여 및 공중보건 프로그램
- 진단적 개입이 필요한 다중 만성 질환을 가진 고령 인구 증가
- 외래 내시경 검사량 증가를 촉진하는 외래 수술 센터 확장
- 시장 성장 억제요인
- 지속적인 감염 관리의 과제와 강화된 규제 모니터링
- 전 세계적 내시경 전문의 및 지원 인력 부족
- 제품 출시 지연시키는 길고 엄격한 규제 승인 절차
- 고급 내시경 시스템의 높은 자본 비용과 수명주기 유지 보수 비용
- 공급망 분석
- 기술의 전망
- Porter's Five Forces
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계
제5장 시장 규모와 성장 예측
- 장비 유형별
- 내시경
- 경성 내시경
- 연성 내시경
- 캡슐 내시경
- 로봇 지원 내시경
- 내시경 수술 장비
- 관개/흡입 시스템
- 접근 장비
- 상처 보호구
- 팽창 장비
- 수동 장비
- 영상 장비
- 내시경 카메라
- SD 영상 시스템
- HD / 4K 영상 시스템
- 내시경
- 용도별
- 위장 내시경
- 복강경 검사
- 호흡기과/기관지경검사
- 이비인후과
- 비뇨기과
- 부인과
- 심장병학
- 신경학
- 정형외과/관절경검사
- 사용성별
- 재처리/재사용 가능한 장비
- 일회용 장비
- 멸균 및 재처리 서비스
- 최종 사용자별
- 병원 및 대학 의료 센터
- 외래수술센터(ASC)
- 전문 클리닉
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 시장 집중도
- 시장 점유율 분석
- 기업 프로파일
- Olympus Corporation
- Boston Scientific Corporation
- Medtronic PLC
- Fujifilm Holdings Corporation
- Karl Storz SE & Co. KG
- Stryker Corporation
- Johnson & Johnson(Ethicon Endo-Surgery)
- Hoya Corporation(Pentax Medical)
- CONMED Corporation
- Richard Wolf GmbH
- Cook Group Incorporated
- Smith & Nephew PLC
- Intuitive Surgical Inc.
- Ambu A/S
- Arthrex Inc.
- B. Braun Melsungen AG
- Ackermann Instrumente GmbH
- Steris PLC
- Interscope Inc.
제7장 시장 기회와 장래의 전망
HBR 25.11.14The endoscopy devices market stands at USD 40.10 billion in 2025 and is forecast to reach roughly USD 55.09 billion by 2030, reflecting a compound annual growth rate (CAGR) close to 6.56%.
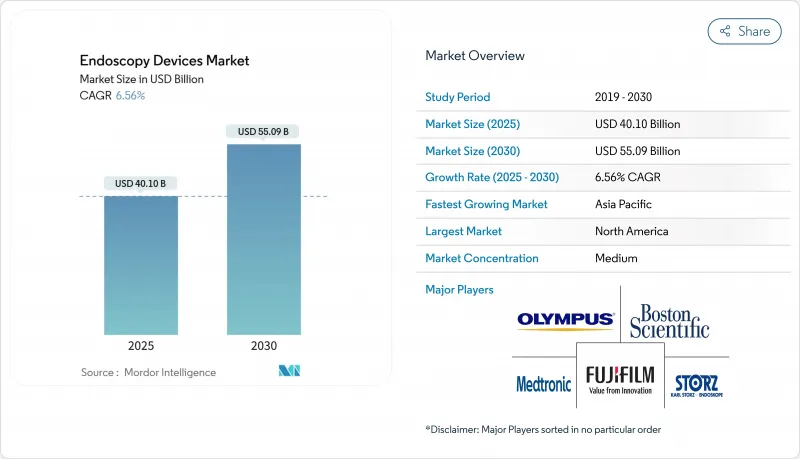
Rising acceptance of minimally invasive surgery continues to reshape hospital capital budgets, drawing investment toward visualization towers and advanced imaging modules that shorten patient recovery times while freeing operating-room slots for high-margin procedures. At the same time, infection-control mandates are redirecting procurement toward disposable or partly disposable scopes, a shift that not only reduces reprocessing labor but also limits reimbursement penalties linked to hospital-acquired infections. Growth in Asia-Pacific is outpacing global averages as expanding insurance coverage collides with a shortage of trained endoscopists, prompting buyers in that region to favor intuitive, software-guided platforms that compress learning curves. Established North American providers remain anchor customers, yet their quick adoption of single-use duodenoscopes is pressuring manufacturers to balance premium pricing with volume-driven cost efficiencies.
Global Endoscopy Devices Market Trends and Insights
Widespread Shift Toward Minimally Invasive Procedures Across Surgical Specialties
Across specialties, clinicians are reshaping care pathways around minimally invasive interventions. Endoluminal robotics-allowing navigation through natural orifices-illustrates how product roadmaps are converging on the same value proposition: lower patient trauma, shorter admissions, and quicker recoveries. A second-order implication is that hospitals now tie capital-budget requests for imaging suites and OR renovations to claims of throughput gains driven by these technologies; therefore, endoscopy vendors must increasingly compete on measurable workflow efficiencies rather than image quality alone.
Continuous Innovation in Endoscopy Visualization Enhancing Clinical Outcomes
High-definition optics, 3D imagery, and software-enhanced contrast platforms have improved early-lesion detection. Olympus's EVIS EXERA III, for example, leverages Narrow Band Imaging (NBI) and Dual Focus to lift adenoma detection rates during colonoscopy. The strategic corollary for providers is the emerging ability to selectively biopsy smaller tissue volumes, which not only accelerates pathology turnaround but also reduces consumable costs at scale. That optimization, in turn, allows outpatient facilities to maintain competitive reimbursement rates while still meeting quality benchmarks.
Global Shortage of Trained Endoscopists and Support Staff
Expanding indications have outpaced the global supply of skilled endoscopists. The American Society for Gastrointestinal Endoscopy stresses that advanced procedures such as ERCP and EUS require prolonged mentorships, deterring rapid credentialing. A pragmatic consequence for suppliers is an emerging market for systems with integrated guidance software, standard-of-care checklists, and artificial-intelligence-assisted navigation. Devices that shorten training timelines improve utilization rates, thereby strengthening the business case for administrators deciding between competing capital requests.
Other drivers and restraints analyzed in the detailed report include:
- Favorable Reimbursement & Public-Health Programs Supporting Preventive Endoscopy
- Rising Global Incidence of Gastrointestinal & Colorectal Cancers Driving Screening Demand
- Persistent Infection-Control Challenges and Heightened Regulatory Scrutiny
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Gastrointestinal endoscopy secured 54.9% market share in 2024, reflecting heavy reliance on colonoscopy and upper-GI diagnostics. Yet laparoscopy is projected to rise at an 8.9% CAGR through 2030, driven by expanded indications such as bariatric revisions and endometriosis treatment. This momentum suggests that multi-disciplinary OR teams may push for modular tower configurations capable of switching between laparoscopic and endoscopic modes, effectively bifurcating procurement strategies into high-volume GI suites versus flexible multi-specialty rooms.
Endoscopic ultrasound (EUS) is also gaining traction, evidenced by rising upper-GI EUS volumes in the United States. Because EUS facilitates both staging and therapeutic interventions like celiac plexus neurolysis, hospitals have started to position the technology as a revenue-enhancing adjunct rather than a diagnostic cost center, subtly shifting budgeting authority from radiology to GI departments.
Reusable scopes still dominate with an 82% share in 2024, but disposables are growing 12.5% annually. Studies comparing disposable-sheath gastroscopes with standard devices demonstrate reprocessing times dropping from roughly 48 minutes to under 10 minutes, effectively tripling possible daily throughput. That throughput improvement is compelling when one considers that most ASCs operate on tight daily schedules; an extra two to three cases per room can materially improve EBITDA margins without extending clinic hours.
The knock-on effect is a heightened focus on waste management and sustainability, as facility managers weigh infection risk mitigation against environmental impact. This dual consideration is likely to influence future purchasing criteria toward recyclable materials and take-back programs, introducing new service-line revenue models for manufacturers.
The Endoscopy Devices Market Report is Segmented by Device Type (Endoscopes [Rigid Endoscopes, and More], Endoscopic Operative Devices [Access Devices, and More], and More), Application (Gastrointestinal, and More), Usability (Reprocessed / Reusable Devices, and More), End-User (Hospitals, Academic Medical Centers, and More) and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
List of Companies Covered in this Report:
- Olympus
- Boston Scientific
- Medtronic
- FUJIFILM
- Karl Storz
- Stryker
- Johnson & Johnson (Ethicon Endo-Surgery)
- HOYA
- Conmed
- Richard Wolf
- Cook Group
- Smiths Group
- Intuitive Surgical
- Ambu
- Arthrex
- B. Braun
- Ackermann Instrumente
- STERIS
- Interscope Inc.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Global Incidence of Gastrointestinal & Colorectal Cancers Driving Screening Demand
- 4.2.2 Widespread Shift Toward Minimally Invasive Procedures Across Surgical Specialties
- 4.2.3 Continuous Innovation in Endoscopy Visualization Enhancing Clinical Outcomes
- 4.2.4 Favorable Reimbursement & Public-Health Programs Supporting Preventive Endoscopy
- 4.2.5 Aging Population with Multiple Chronic Conditions Requiring Diagnostic Interventions
- 4.2.6 Expansion of Ambulatory Surgical Centers Boosting Outpatient Endoscopy Volumes
- 4.3 Market Restraints
- 4.3.1 Persistent Infection-Control Challenges and Heightened Regulatory Scrutiny
- 4.3.2 Global Shortage of Trained Endoscopists and Support Staff
- 4.3.3 Lengthy, Stringent Regulatory Approval Processes Slowing Product Launches
- 4.3.4 High Capital & Lifecycle Maintenance Costs of Advanced Endoscopic Systems
- 4.4 Supply-Chain Analysis
- 4.5 Technological Outlook
- 4.6 Porter's Five Forces
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitutes
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Device Type
- 5.1.1 Endoscopes
- 5.1.1.1 Rigid Endoscopes
- 5.1.1.2 Flexible Endoscopes
- 5.1.1.3 Capsule Endoscopes
- 5.1.1.4 Robotic-assisted Endoscopes
- 5.1.2 Endoscopic Operative Devices
- 5.1.2.1 Irrigation / Suction Systems
- 5.1.2.2 Access Devices
- 5.1.2.3 Wound Protectors
- 5.1.2.4 Insufflation Devices
- 5.1.2.5 Manual Instruments
- 5.1.3 Visualization Equipment
- 5.1.3.1 Endoscopic Cameras
- 5.1.3.2 SD Visualization Systems
- 5.1.3.3 HD / 4K Visualization Systems
- 5.1.1 Endoscopes
- 5.2 By Application
- 5.2.1 Gastrointestinal Endoscopy
- 5.2.2 Laparoscopy
- 5.2.3 Pulmonology / Bronchoscopy
- 5.2.4 ENT / Otolaryngology
- 5.2.5 Urology
- 5.2.6 Gynecology
- 5.2.7 Cardiology
- 5.2.8 Neurology
- 5.2.9 Orthopedics / Arthroscopy
- 5.3 By Usability
- 5.3.1 Reprocessed / Reusable Devices
- 5.3.2 Single-use / Disposable Devices
- 5.3.3 Sterilisation & Reprocessing Services
- 5.4 By End-User
- 5.4.1 Hospitals & Academic Medical Centers
- 5.4.2 Ambulatory Surgical Centers
- 5.4.3 Specialty Clinics
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Olympus Corporation
- 6.3.2 Boston Scientific Corporation
- 6.3.3 Medtronic PLC
- 6.3.4 Fujifilm Holdings Corporation
- 6.3.5 Karl Storz SE & Co. KG
- 6.3.6 Stryker Corporation
- 6.3.7 Johnson & Johnson (Ethicon Endo-Surgery)
- 6.3.8 Hoya Corporation (Pentax Medical)
- 6.3.9 CONMED Corporation
- 6.3.10 Richard Wolf GmbH
- 6.3.11 Cook Group Incorporated
- 6.3.12 Smith & Nephew PLC
- 6.3.13 Intuitive Surgical Inc.
- 6.3.14 Ambu A/S
- 6.3.15 Arthrex Inc.
- 6.3.16 B. Braun Melsungen AG
- 6.3.17 Ackermann Instrumente GmbH
- 6.3.18 Steris PLC
- 6.3.19 Interscope Inc.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment

















