
|
시장보고서
상품코드
1444956
핵산 분리, 정량 및 정제 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Nucleic Acid Isolation, Quantitation, and Purification - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
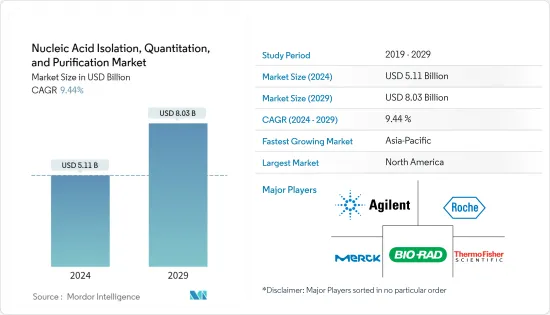
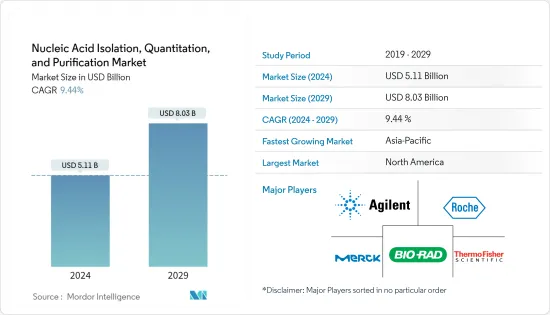
핵산 분리, 정량 및 정제 시장 규모는 2024년 51억 1,000만 달러로 추정되며 2029년까지 80억 3,000만 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 9.44%의 CAGR로 성장할 것으로 예상됩니다.
COVID-19는 COVID-19 검출을 위한 핵산 스크리닝의 급증으로 인한 시약 및 장비 부족 등 다양한 요인으로 인해 초기 단계에서 핵산 분리, 정량 및 정제 시장에 큰 영향을 미쳤습니다. 예를 들어, 2022년 1월 Frontiers에 게재된 기사에 따르면, 팬데믹 기간 동안 중국 구룡포의 한 지역에서는 총 7,118명이 10,377번의 SARS-CoV-2 핵산 검사를 받았다고 합니다. 또한, 2022년 10월 PubMed는 무증상 감염 사례의 증가로 인해 일반 인구의 정기적인 핵산 검사가 실질적이고 효과적인 예방 및 통제 수단으로 발전했다고 밝혔습니다. 대유행 이후 상황에서 핵산의 분리, 정량 및 분리를 위해 많은 첨단 기술이 사용되고 있으며 더 많은 시약과 장비에 대한 수요가 증가할 것으로 예상됩니다. 예를 들어, 2022년 5월 Springer 저널에 게재 된 논문에 따르면 연구원들은 바이오의약품 제조의 정제 과정에서 발생하는 문제를 해결하기 위해 아미노산이 특정 리간드 역할을하는 친 화성 크로마토그래피를 사용하여 플라스미드 DNA를 정제 할 것을 제안했습니다. 제안하였습니다. 따라서 게놈 연구의 발전에 따른 핵산 분리, 정량 및 정제에 대한 연구 활동이 증가함에 따라 시장이 성장할 것으로 예상됩니다.
임상 진단에서 시퀀싱 플랫폼에 대한 수요 증가, 유전체학 연구 증가, 분자생물학 연구 및 개발 자금 증가 등의 요인이 예측 기간 동안 시장 성장을 견인할 것으로 예상됩니다. 예를 들어, 2022년 10월 첸나이에 본사를 둔 신생 기업 MagGenome은 DNA 추출을 용이하게 하는 새로운 기술을 도입했습니다. 절차는 간단하고 명확하며, 철 나노입자를 생성하여 DNA에 결합을 유도하고 자기장을 적용하여 단백질과 RNA를 모두 추출합니다. 또한, 핵산 정량에 초점을 맞춘 연구는 예측 기간 동안 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, 2022년 9월 MDPI 저널에 게재된 기사에 따르면 정확한 DNA 정량는 분자 생물학에서 매우 중요한 방법이며, UV 분광 분석과 형광 분석은 DNA 정량에 널리 사용되고 있습니다. 이 연구는 시료의 순도와 dsDNA 함량에 대한 정보를 제공하기 위해 분광 광도법과 형광 분석법을 결합하는 것이 권장된다는 것을 입증했습니다. 따라서 연구에 더 많은 핵산 추출, 정량 및 분리 제품이 필요할 수 있으며, 이는 예측 기간 동안 시장 성장을 촉진할 것입니다.
또한, 핵산 분리 및 정제와 관련된 연구 분야의 기술 발전은 예측 기간 동안 시장 성장을 촉진할 수 있습니다. 예를 들어, Taylor and Francis Online에 2022년 4월에 게재된 기사에 따르면, 시중에서 판매되는 대부분의 핵산 분리 키트는 실리카 기반입니다. 이 연구에서 연구원들은 핵산 정제를위한 실리카 기반 프로토콜을 향상시키는 새로운 방법에 중점을 두었습니다. 이 연구는 실리카 재료를 기반으로 한 혁신적인 정제 기술로 해결해야 할 현재 요구 사항을 강조하는 것을 목표로했습니다.
또한, 주요 기업들의 제품 출시와 협업으로 시장 경쟁이 더욱 치열해질 것으로 예상됩니다. 예를 들어, 생체 샘플에서 핵산을 추출하고 정제하는 차세대 기술을 제공하는 퓨리젠 바이오시스템즈(Purigen Biosystems, Inc.)는 2022년 4월 Ionic Cells to Pure DNA Kit를 출시했습니다. 이 새로운 키트는 이온 정제 시스템 사용자가 WBC, 말초혈액 단핵구(PBMC) 및 배양 또는 선별된 세포에서 더 많은 고품질 DNA를 추출할 수 있도록 최적화되어 있습니다.
따라서 핵산 분리, 정량 및 정제 분야의 연구 활동, 자금 조달 및 시약 제품 출시가 증가함에 따라 핵산 분리, 정량 및 정제 시장은 예측 기간 동안 성장할 것으로 예상됩니다. 그러나 높은 장비 및 시약 비용과 신흥 시장에서의 낮은 보급률로 인해 예측 기간 동안 시장 성장이 억제될 것으로 예상됩니다.
핵산 분리 및 정제 시장 동향
DNA 정량 키트 부문은 예측 기간 동안 성장할 것으로 예상
DNA 정량 키트 부문은 DNA 정량 키트 제품 출시 증가, 게놈 연구 증가, 연구 자금 급증 등의 요인으로 인해 핵산 분리, 정량, 정제 시장의 성장이 예상됩니다. 핵산 정량 및 분리가 포함됩니다. DNA 정량 키트는 분리된 DNA 샘플의 양과 품질을 종합적으로 평가합니다. 이는 다운스트림 처리와 관련하여 올바른 결정을 내리는 데 도움이되므로 각 샘플의 성공 가능성을 높일 수 있습니다. 따라서 DNA 정량 키트는 일반적으로 샘플 양이 많고 오류를 최소화할 수 있는 법의학 연구소나 실험실에서 유리한 선택이 될 수 있습니다.
또한, DNA 정량 키트의 장점으로는 향상된 특이성, 높은 동적 범위를 갖춘 견고하고 재현성이 높은 제품, 낮은 결과 노이즈 등이 있으며, DNA 정량의 중요성과 이점을 입증하는 연구 활동이 증가함에 따라 예측 기간 동안 이 부문의 성장은 기존보다 더 높아질 것으로 예상됩니다. 증가할 것으로 예상됩니다. 예를 들어, 2022년 9월 ScienceDirect에 게재된 기사에 따르면, 이 연구는 Quantifiler Trio DNA Quantification Kit 및 Investigator Quantiplex Pro RGQ Kit의 마커의 기초가 되는 DNA 농도 측정을 분석했습니다. 사용 된 측정 키트에 따라 짧은 상염색체 단편의 DNA 농도 측정을 기반으로 80 개 중 12 개(Quantifiler Trio DNA Quantification Kit) 또는 80 개 중 11 개(Investigator Quantiplex Pro RGQ)가 STR 증폭 키트의 제조업체 요구 사항을 달성했습니다. 제조업체의 요구량을 달성했습니다. 반응 혼합물 내 DNA의 양. 따라서 DNA 정량 키트를 포함한 이러한 연구는 예측 기간 동안 부문의 성장을 촉진할 것으로 예상됩니다.
또한, DNA 정량 관련 연구에 대한 연구 자금은 예측 기간 동안 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, 2022년 11월, 플로리다 대학교 의과대학의 연구진은 DNA의 복구 메커니즘을 연구하기 위해 국립 일반 의학 연구소로부터 180만 달러의 보조금을 받았습니다. 또한 주요 기업의 DNA 정량 제품 출시는 예측 기간 동안 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, 2022년 7월 Qiagen은 QIAcuity 디지털 PCR 포트폴리오에 새로운 바이오의약품 제품을 추가하고 Expert Custom Assay Design Service를 출시했습니다. 13개의 새로운 키트와 분석법을 통해 세포 치료 및 유전자 치료에서 AAV 바이러스 역가 및 잔류 숙주 세포 DNA를 정량할 수 있습니다. 숙주 세포 DNA의 이월을 확인하기 위해 세 가지 새로운 QIAcuity 잔류 DNA 정량 키트를 사용할 수 있습니다.
따라서, 제품 출시의 증가와 연구 활동의 증가로 인해 DNA 정량 키트 부문은 예측 기간 동안 핵산 분리, 정량 및 정제 시장의 성장을 보일 것으로 예상됩니다.
북미는 예측 기간 동안 시장에서 상당한 점유율을 차지할 것으로 예상
북미에서는 핵산 분리, 정량, 정제 절차가 필요한 게놈 분야의 연구개발 증가와 시퀀싱 수요 증가로 인해 핵산 분리, 정량, 정제 시장이 성장할 것으로 예상됩니다. 임상 진단 플랫폼, 분자생물학 연구개발에 대한 자금 지원 증가. 예를 들어, 2022년 12월 맥마스터는 캐나다의 소형 모듈형 원자로 연구 개발부터 의료용 동위원소 접근성 개선, 중성자선 과학 발전 등 다양한 프로젝트에서 핵과학 분야 파트너들과 협력했습니다.
또한 2022년 5월, QIAGEN은 현재 알려진 모든 활성 EGFR 돌연변이 및 내성 EGFR 돌연변이를 검출하는 고감도 EGFR 돌연변이 분석을 위한 새로운 체외 진단 테스트인 therascreen EGFR Plus RGQ PCR Kit를 출시할 예정입니다. 실시간 qPCR 테스트는 기존의 테라스크린 EGFR RGQ PCR 키트를 기반으로 구축됐습니다. 향상된 검출 한계, 단축된 소요 시간, 자동 샘플 추출 옵션 및 자동 결과 분석이 제공됩니다. 핵산 검출 및 정제의 이러한 발전은 시장 성장을 촉진할 것으로 예상됩니다. 또한 2021년 9월, Beckman Coulter Life Sciences는 PCR 정제에 필요한 시간을 단축하고 워크플로우를 반자동화하는 EMnetik 시스템을 출시하였습니다. 전자석을 사용하여 구축된 EMnetik 24와 함께 제공되는 EMnetik PCR Cleanup Kit 및 EMnetik Plasmid Purification Kit는 마그네틱 비드 기반 기술을 사용하여 고정된 장치 내의 핵산을 세척합니다. 벤치탑 장비는 한 번에 1-24개의 샘플을 실행할 수 있습니다. 또한 간소화된 클린업 프로세스와 반자동화를 통해 잠재적인 오류를 제거할 수 있습니다.
따라서 핵산 분리, 정량 및 정제 절차가 필요한 게놈 분야의 연구 개발 및 제품 출시가 급증함에 따라 북미 지역의 핵산 분리 및 정제 시장은 측정 기간 동안 성장할 것으로 예상됩니다. 예
핵산 분리 및 정제 산업 개요
핵산 분리, 정량 및 정제 시장은 세계 및 지역적으로 운영되는 주요 기업의 존재로 인해 본질적으로 통합되어 있습니다. 경쟁 환경에는 시장 점유율이 큰 여러 국제 및 현지 기업의 분석이 포함되어 있습니다. 주요 업체로는 Bio-Rad Laboratories Inc., Thermo Fisher Scientific, Inc., Merck KGaA, F. Hoffmann-La Roche AG, Agilent Technologies 등이 있습니다.
기타 혜택
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간 애널리스트 지원
목차
제1장 서론
- 조사 가정과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 임상 진단의 시퀀싱 플랫폼 수요 증가
- 유전체학 조사의 대두
- 분자생물학 연구개발에 대한 자금 증가
- 시장 성장 억제요인
- 기기와 시약 가격이 높은
- 신흥 시장에서의 보급률 낮음
- Porter's Five Forces 분석
- 신규 참여업체의 위협
- 구매자의 교섭력
- 공급 기업의 교섭력
- 대체 제품의 위협
- 경쟁 기업간 경쟁 강도
제5장 시장 세분화
- 기술별
- 컬럼 기반 정제
- 자기 비즈 기반 정제
- 시약 기반 정제
- 제품별
- 키트와 시약
- 핵산 분리와 정제
- 핵산 정량
- DNA 정량 키트
- RNA 정량 키트
- 설비
- 핵산 분리와 정제
- 핵산 정량
- 분광광도계
- 형광 광도계
- 기타 제품
- 키트와 시약
- 용도별
- 토탈 RNA 분리와 정제
- mRNA 분리와 정제
- 마이크로 RNA 분리와 정제
- 플라스미드 DNA 분리와 정제
- 게놈 DNA 분리와 정제
- 혈액 DNA 분리와 정제
- PCR 클린 업
- 바이오뱅크
- 임상 조사
- 법의학
- 의약품 개발
- 기타
- 최종사용자별
- 병원
- 학술계
- 제약/바이오테크놀러지 산업
- CRO
- 지역
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 상황
- 기업 개요
- Agilent Technologies
- Illumina, Inc
- QIAGEN
- Bio-Rad Laboratories Inc.
- Danaher Corporation(Beckman Coulter)
- General Electric Company(GE Healthcare)
- F. Hoffmann-La Roche Ltd
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Takara Holdings
- Promega Corporation
- PerkinElmer
- BioVision Inc.
제7장 시장 기회와 향후 동향
ksm 24.03.18The Nucleic Acid Isolation, Quantitation, and Purification Market size is estimated at USD 5.11 billion in 2024, and is expected to reach USD 8.03 billion by 2029, growing at a CAGR of 9.44% during the forecast period (2024-2029).
COVID-19 significantly impacted the nucleic acid isolation, quantitation, and purification market during its initial phase owing to various factors, such as the shortage of reagents and equipment due to the surge in nucleic acid screening for COVID-19 detection. For instance, as per the article published in January 2022 in Frontiers, a total of 7,118 people received 10,377 SARS-CoV-2 nucleic acid tests in one district of Jiulongpo district of China during the pandemic. Furthermore, in October 2022 in PubMed, routine nucleic acid screening in the general population evolved into a substantial and effective prevention and control measure as a result of the increased number of asymptomatic infection cases. Numerous advanced techniques are being used in the post-pandemic setting for nucleic isolation, quantification, and separation, which is expected to increase the demand for more reagents and equipment. For instance, as per the article published in May 2022 in Springer, researchers suggested the purification of Plasmid DNA using affinity chromatography, with amino acids acting as particular ligands to rectify the challenges associated with the purification process in biopharmaceutical manufacturing. Thus, the market is anticipated to witness growth due to the rise in research activities in nucleic acid isolation, quantitation, and purification along with an increase in development in genomic research.
Factors such as an increase in demand for sequencing platforms in clinical diagnostics, a rise in genomics research, and growth in funding for research and development in molecular biology are anticipated to drive the market growth over the forecast period. For instance, in October 2022, Chennai-based start-up MagGenome introduced a new technology that makes DNA extraction easy. The procedure is simple and clear, which creates iron nanoparticles, induces them to bind to DNA, and then applies a magnetic field to extract both proteins and RNA. Furthermore, research studies focusing on nucleic acid quantitation are expected to boost market growth over the forecast period. For instance, as per the article published in September 2022 in the MDPI journal, accurate DNA quantification is a very important method within molecular biology and UV spectrometry and fluorometry are widely used to quantify DNA. The study demonstrated that combining a spectrophotometric and fluorometric approach is advised to provide information on a sample's purity and dsDNA content. Thus, research is likely to require more nucleic acid extraction, quatitation and isolation products, thereby driving market growth over the forecast period.
Furthermore, the technological advancements in the research associated with the isolation and purification of nucleic acid are likely to boost market growth over the forecast period. For instance, as per the article published in April 2022 in Taylor and Francis Online, the majority of commercial nucleic acid isolation kits available are silica-based. In the study, researchers focused on new methods to enhance silica-based protocols for nucleic acid purification. The study intended to highlight the current needs to be addressed by innovative purification techniques based on silica materials.
Furthermore, product launches and collaboration by the key players are anticipated to strengthen the competition in the market. For instance, in April 2022, Purigen Biosystems, Inc., a provider of next-generation technologies for extracting and purifying nucleic acids from biological samples, launched the Ionic Cells to Pure DNA Kit. The new kit was optimized to allow users of the Ionic Purification System to extract increased yields of high-quality DNA from WBCs, peripheral blood mononuclear cells (PBMCs), and cultured or sorted cells.
Hence, due to the rise in research activities, funding in the field of nucleic acid isolation, quantitation, and purification, and an increase in reagent product launches, the nucleic acid isolation quantitation, and purification market is anticipated to witness growth over the forecast period. However, the high cost of instruments and reagents and low penetration in Emerging markets are expected to restrain the market growth over the forecast period.
Nucleic Acid Isolation & Purification Market Trends
DNA Quantitation Kits Segment is Anticipated to Witness a Growth Over the Forecast Period
The DNA quantitation kits segment is expected to witness growth in the nucleic acid isolation, quantitation, and purification market, owing to the factors such as a rise in DNA quantitation kit product launches, an increase in genomic research, and a surge in funding for research involving nucleic acid quantitation and isolations. DNA quantitation kits provide a comprehensive assessment of the quantity and quality of the isolated DNA sample. This is helpful in making the correct decisions regarding downstream processing, therefore, improving room for success with each sample. This makes DNA quantitation kits a lucrative option in forensic laboratories and labs, where high sample volume is generally low and there is minimal room for error.
Furthermore, the benefits of DNA quantitation kits include improved specificity, robust and reproducible products with a high dynamic range, low noise in the results, etc. The rise in research activities demonstrating the importance and advantages of DNA quantitation is anticipated to increase segment growth over the forecast period. For instance, as per the article published in September 2022 in ScienceDirect, the study analyzed DNA concentration measurement which resulted in the basis of markers of the Quantifiler Trio DNA Quantification Kit and Investigator Quantiplex Pro RGQ Kit. Depending on the measurement kit used, based on measuring the concentration of DNA of the short autosomal fragment, 12 out of 80 (Quantifiler Trio DNA Quantification Kit) or 11 out of 80 (Investigator Quantiplex Pro RGQ) achieved the STR amplification kit manufacturer's required amount of DNA in the reaction mixture. Hence, such studies involving DNA quantitation kits are expected to drive segment growth over the forecast period.
Moreover, research funding for the studies associated with DNA quantitation is anticipated to drive market growth over the forecast period. For instance, in November 2022, a research scholar at the University of Florida College of Medicine was awarded a USD 1.8 million grant from the National Institute of General Medical Sciences to study the repair mechanism of DNA. Additionally, the DNA quantitation product launches by the key players are anticipated to boost market growth over the forecast period. For instance, in July 2022, Qiagen added new biopharma products to its QIAcuity digital PCR portfolio and launched its Expert Custom Assay Design Service. Thirteen new kits and assays allow for the quantification of AAV viral titer and residual host cell DNA in cell and gene therapy. Three new QIAcuity Residual DNA Quantification Kits are available for checking the carryover of the host cell DNA.
Hence, due to the rise in product launches and increased research activities, the DNA quantitation kit segment is anticipated to witness growth in the nucleic acid isolation, quantitation, and purification market over the forecast period.
North America Anticipated to Hold a Significant Share in the Market Over the Forecast Period
North America is expected to witness growth in the nucleic acid isolation quantitation and purification market owing to the factors such as a rise in research and development in the genomic field that requires nucleic acid isolation, quantitation, and purification procedures, an increase in demand for sequencing platforms in clinical diagnostics, and growth in funding for research and development in molecular biology. For instance, in December 2022, McMaster collaborated with partners in the field of nuclear science on a range of projects, from small modular reactor research and development to improving access to medical isotopes and advancing neutron beam science in Canada.
In addition, in May 2022, QIAGEN launched its therascreen EGFR Plus RGQ PCR Kit, a new in-vitro diagnostic test for sensitive EGFR mutation analysis, detecting all currently known activating and resistance EGFR mutations. The real-time qPCR test builds on the established therascreen EGFR RGQ PCR Kit. It provides improved detection limits, quicker turnaround times, automated sample extraction options, and automated results analysis. Such developments in nucleic acid detection and purification are anticipated to drive market growth. Furthermore, in September 2021, Beckman Coulter Life Sciences launched the EMnetik System to semi-automate workflows while reducing the time required for a PCR cleanup. Built using electromagnets, EMnetik 24 and the accompanying EMnetik PCR Cleanup Kit and EMnetik Plasmid Purification Kit use magnetic bead-based technology to clean nucleic acids in a stationary device. The benchtop instrument can run 1-24 samples at a time. The streamlined cleanup process and semi-automation also helped eliminate potential errors.
Thus, due to the rise in research and development in the genomic field that requires nucleic acid isolation, quantitation, and purification procedures, along with the surge in product launches, North America is anticipated to witness a growth in the nucleic acid isolation and purification market over the forecast period.
Nucleic Acid Isolation & Purification Industry Overview
The nucleic acid isolation quantitation and purification market is consolidated in nature due to the presence of major companies operating globally as well as regionally. The competitive landscape includes an analysis of a few international and local companies with significant market shares. Major players include Bio-Rad Laboratories Inc., Thermo Fisher Scientific, Inc., Merck KGaA, F. Hoffmann-La Roche AG, and Agilent Technologies, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope Of The Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increase in Demand of Sequencing Platforms in Clinical Diagnostics
- 4.2.2 Rise in Genomics Research
- 4.2.3 Growth in Funding for Research and Development in Molecular Biology
- 4.3 Market Restraints
- 4.3.1 High Price of the Instruments and Reagents
- 4.3.2 Low Penetration in Emerging Markets
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD Million)
- 5.1 By Technology
- 5.1.1 Column-based Purification
- 5.1.2 Magnetic Bead-based Purification
- 5.1.3 Reagent-based Purification
- 5.2 By Product
- 5.2.1 Kits and Reagents
- 5.2.1.1 Nucleic Acid Isolation and Purification
- 5.2.1.2 Nucleic Acid Quantitation
- 5.2.1.2.1 DNA Quantitation Kits
- 5.2.1.2.2 RNA Quantitation Kits
- 5.2.2 Equipments
- 5.2.2.1 Nucleic Acid Isolation and Purification
- 5.2.2.2 Nucleic Acid Quantitation
- 5.2.2.2.1 Spectrophotometer
- 5.2.2.2.2 Fluorometer
- 5.2.3 Other Products
- 5.2.1 Kits and Reagents
- 5.3 By Application
- 5.3.1 Total RNA Isolation and Purification
- 5.3.2 mRNA Isolation and Purification
- 5.3.3 microRNA Isolation and Purification
- 5.3.4 Plasmid DNA Isolation and Purification
- 5.3.5 Genomic DNA Isolation and Purification
- 5.3.6 Blood DNA Isolation and Purification
- 5.3.7 PCR Clean-up
- 5.3.8 Biobanking
- 5.3.9 Clinical Research
- 5.3.10 Forensics
- 5.3.11 Drug Development
- 5.3.12 Others
- 5.4 By End-user
- 5.4.1 Hospitals
- 5.4.2 Academia
- 5.4.3 Pharmaceutical/Biotechnology Industry
- 5.4.4 CRO
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Agilent Technologies
- 6.1.2 Illumina, Inc
- 6.1.3 QIAGEN
- 6.1.4 Bio-Rad Laboratories Inc.
- 6.1.5 Danaher Corporation (Beckman Coulter)
- 6.1.6 General Electric Company (GE Healthcare)
- 6.1.7 F. Hoffmann-La Roche Ltd
- 6.1.8 Merck KGaA
- 6.1.9 Thermo Fisher Scientific Inc.
- 6.1.10 Takara Holdings
- 6.1.11 Promega Corporation
- 6.1.12 PerkinElmer
- 6.1.13 BioVision Inc.

















