
|
시장보고서
상품코드
1445459
병리 기기 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Pathology Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
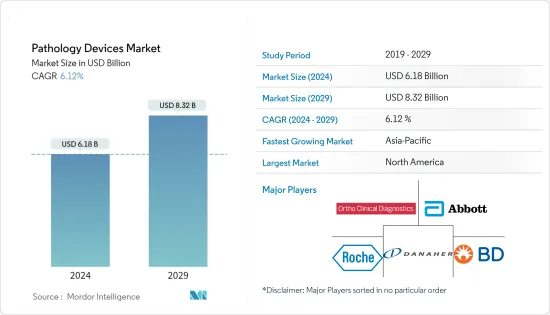
병리 기기 시장 규모는 2024년 61억 8,000만 달러로 추정되며, 2029년까지 83억 2,000만 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 6.12%의 CAGR로 성장할 것으로 예상됩니다.
주요 하이라이트
- COVID-19는 전 세계 의료 시스템에 큰 영향을 미쳤으며, 많은 의료 시설이 일상적인 진료 및 병리 검사를 중단하여 암 및 심혈관 질환과 같은 만성 질환을 앓고 있는 환자를 심각한 위험에 노출시켰습니다. 예를 들어, 2022년 1월 International Medical Journal에 게재된 논문에 따르면, 봉쇄 전과 봉쇄 후 병리 검사 횟수와 기준 기간의 비율이 각각 1.02에서 0.53으로 감소한 것으로 나타났습니다.
- 그러나 국민들 사이에서 코로나 바이러스 감염자가 증가함에 따라 병리학 검사에 대한 수요가 증가하고 있습니다. 예를 들어, 2022년 3월 Frontiers in Pathology에 게재된 기사에 따르면, 봉쇄 기간 동안 분자 미생물 검사 건수가 3배 이상 증가한 것으로 나타났습니다. 이로 인해 회사는 혁신적인 병리학 검사 키트 및 장비 개발에 더욱 집중하게 되었습니다. 따라서 조사 대상 시장은 향후 몇 년 동안 성장하여 잠재력을 최대한 발휘할 것으로 예상됩니다.
- 전 세계적으로 알츠하이머병, 자가 면역 질환, 암 등의 유병률과 부담이 증가함에 따라 효과적이고 진보된 진단 테스트에 대한 필요성이 증가하여 시장 성장을 주도하고 있습니다. 예를 들어, 알츠하이머병 협회가 발표한 2022년 통계에 따르면 2022년 65세 이상 미국인 중 약 650만 명이 알츠하이머병을 앓고 있으며, 이 숫자는 2050년까지 1,300만 명에 달할 것으로 예측됩니다. 알츠하이머병 환자 비율이 증가함에 따라 의사가 질병을 유발하는 치매 증상을 조기에 식별하는 데 도움이 되는 병리학적 장치에 대한 수요가 증가하여 시장 성장을 촉진할 것으로 예상됩니다.
- 또한 ACS가 발표한 2023년 통계에 따르면 2023년 미국에서 약 24,810건의 뇌 또는 척수 악성 종양(남성 14,280건, 여성 10,530건)이 진단될 것으로 예상됩니다. 척수 종양은 척추의 병리학적 상태를 감지하기 위한 방사성 핵종 골 신티그래피(뼈 스캔)에 대한 수요를 자극하여 시장 성장을 촉진할 것으로 예상됩니다. 또한, 기술적으로 진보된 병리학적 키트 및 장비 개발에 주력하는 신생 기업들도 시장 성장에 기여하고 있습니다. 예를 들어, 2022년 4월 시스멕스 유럽은 새로운 3부 구성의 차동 시스템인 XQ-320 XQ 시리즈 자동 혈액 분석기를 출시했습니다. 신뢰할 수있는 기술과 새로운 기능을 통해 다양한 임상 검사 환경에 탁월한 품질을 제공하는 견고한 장치입니다. 사용감 수준.
- 따라서 만성 질환 및 감염성 질환의 높은 부담과 신제품 출시 등의 요인으로 인해 조사 대상 시장은 예측 기간 동안 성장할 것으로 예상됩니다. 그러나 높은 장비 비용과 엄격한 규제, 숙련된 전문가 부족은 예측 기간 동안 병리학 장비의 성장을 저해할 수 있습니다.
병리 기기 시장 동향
분자 진단 부문은 예측 기간 동안 상당한 시장 점유율을 유지할 것으로 예상
- 분자 진단 장치는 게놈과 프로테옴의 생물학적 마커를 분석하여 병원균과 돌연변이를 검출하는 데 사용됩니다. 이 기술을 기반으로 분자 진단 장치는 칩 및 마이크로 어레이, 질량 분석법, 차세대 시퀀싱(NGS), 중합 효소 연쇄 반응(PCR) 기반 방법, 세포 유전학 및 분자 이미징으로 나눌 수 있습니다. 박테리아 및 바이러스 전염병의 대규모 발생, 현장 진단에 대한 수요 증가, 빠르게 발전하는 기술 등의 요인이 분자 진단 분야의 성장을 주도하고 있습니다.
- 암, 심혈관 질환, 뇌종양 등으로 인한 부담 증가는 이 부문의 성장을 촉진하는 중요한 요인으로 작용하고 있습니다. 예를 들어, 영국 심장 재단이 2022년 1월에 발표한 통계에 따르면 2021년 영국에서 760만 명 이상이 심혈관 질환을 앓고 있는 것으로 나타났습니다.
- 또한 미국 뇌종양협회가 발표한 2022년 통계에 따르면 2022년 미국에서는 약 88,970명이 원발성 뇌종양 진단을 받았다고 합니다. 따라서 인구에서 심장병과 뇌종양의 높은 유병률은 뇌종양의 증가를 촉진할 것으로 예상됩니다. 분자 진단에 대한 수요가 증가하고 있으며, 이는 진단 정확도를 높이고, 예후 예측을 가능하게 하며, 표적 식별을 가능하게 합니다. 이는 예측 기간 동안 부문의 성장을 촉진할 것으로 예상됩니다.
- 또한, 분자 진단 분야에서 기술적으로 진보된 제품 개발 및 신제품 출시에 대한 기업의 집중도가 높아지면서 시장 성장에 기여하고 있습니다. 예를 들어, Mylab Discovery Solutions는 2022년 3월에 면역학, 생화학 및 혈액학에 대한 모든 종류의 일상적인 진단 키트와 장치를 기존 및 현장 진료 형태로 출시했습니다. 이 제품군 확장을 통해 실험실은 이 회사의 장치와 시약 키트를 사용하여 간 패널, 심장 프로필, 소변 패널, 호르몬 패널, 발열 패널, 신장 기능 검사, 암 마커 및 기타 테스트와 같은 모든 일상적인 검사를 수행할 수 있습니다.
- 따라서 심장병 및 뇌종양에 대한 높은 부담, 고정밀 의료에 대한 수요 증가, 제품 출시 증가 등의 요인으로 인해 해당 부문은 예측 기간 동안 성장할 것으로 예상됩니다.
북미는 예측 기간 동안 상당한 시장 점유율을 차지할 것으로 예상
- 북미 지역은 감염성 및 만성 질환의 확산, 의료 인프라 개선, 병리 기기 기술 발전으로 인해 병리 기기 시장에서 큰 점유율을 차지할 것으로 예상됩니다.
- 이 지역의 만성 질환 및 감염성 질환의 증가로 인해 병리의 조기 발견 및 진단에 대한 수요가 증가하여 병리 검사에 대한 수요를 자극하여 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, ACS가 발표한 2023년 통계에 따르면, 2023년 미국에서 약 1,958,310명의 신규 암 환자가 진단될 것으로 예상됩니다.
- 또한 같은 자료에 따르면 2023년 미국에서 16만 3,020건의 대장암, 23만 8,340건의 폐 및 기관지암, 29만 7,790건의 유방암, 28만 8,300건의 전립선암이 새로 진단될 것으로 예상했습니다. CDC가 발표한 2022년 자료에 따르면, 2030년에는 미국 내 약 1,210만 명이 심방세동을 앓을 것으로 예상됩니다. 따라서 인구의 유방암과 심장병 부담이 증가함에 따라 진단 검사에 대한 수요가 증가할 것으로 예상됩니다. 암의 조기 발견에 기여하고 시장 성장을 촉진할 것입니다.
- 또한, 이 지역에서 신제품 출시가 증가함에 따라 제품 및 테스트 키트의 가용성이 증가하여 예측 기간 동안 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, 2021년 6월 Thermo Fisher Scientific Inc.는 활성 SARS-CoV-2 감염을 검출하기 위한 새로운 CE-IVD 인증 고속 PCR 콤보 키트 2.0인 TaqPath를 출시했습니다.
- 또한 2021년 3월 MatMaCorp는 중합효소 연쇄반응(PCR) 증폭 및 실시간 형광 검출을 수행할 수 있는 새로운 휴대용 장치 MY Real-Time Analyzer(MYRTA)를 출시했습니다. 따라서 인구의 암 및 심장 질환의 높은 부담, 기업 활동 증가, 지역 내 병리학 연구소의 수 증가와 같은 앞서 언급 한 요인으로 인해 조사 대상 시장은 예측 기간 동안 성장할 것으로 예상됩니다.
병리 기기 산업 개요
병리 기기 시장은 수많은 세계 기업과 로컬 기업이 시장에 존재하기 때문에 세분화되어 있습니다. 두 회사는 시장 지위를 유지하기 위해 제품 출시, 계약, 협업 등 다양한 비즈니스 전략을 채택하는 데 주력하고 있습니다. 시장 주요 기업으로는 Abbott Laboratories, Becton, Dickinson and Company, Ortho-Clinical Diagnostics, F. Hoffmann-La Roche AG, Danaher Corporation, Bio- Rad Laboratories 등이 있습니다. Rad Laboratories 등이 있습니다.
기타 혜택
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간 애널리스트 지원
목차
제1장 서론
- 조사 가정과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 만성질환 및 감염증 이환율 증가
- 병리 디바이스의 기술 진보
- 신흥 국가의 헬스케어 인프라에 대한 투자가 증가
- 시장 성장 억제요인
- 디바이스의 높은 비용
- 엄격한 규제와 숙련된 전문가의 부족
- Porter's Five Forces 분석
- 신규 참여업체의 위협
- 구매자의 교섭력
- 공급 기업의 교섭력
- 대체 제품의 위협
- 경쟁 기업간 경쟁 강도
제5장 시장 세분화
- 기술별
- 임상화학
- 면역측정 기술
- 미생물학
- 분자진단학
- 기타 기술
- 용도별
- Drug Discovery & Development
- 질병 진단
- 법의학 진단
- 기타 용도
- 최종사용자별
- 제약회사
- 병원 및 진단 연구소
- 기타 최종사용자
- 지역
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 상황
- 기업 개요
- Abbott Laboratories
- Becton, Dickinson and Company
- Bio-Rad Laboratories
- Beckman Coulter Inc.
- Definiens
- Hamamatsu Photonics
- Mikroscan Technologies
- Ortho-Clinical Diagnostics
- F. Hoffmann-La Roche AG
- Thermo Fisher Scientific
- Danaher Corporation
- Siemens Healthineers
제7장 시장 기회와 향후 동향
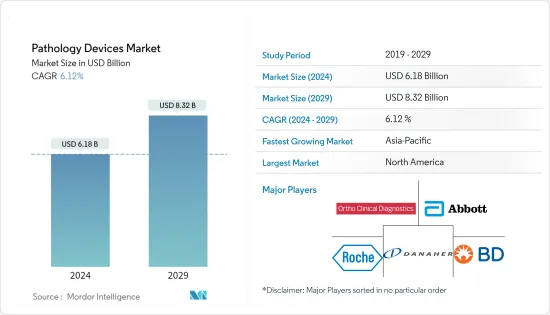
ksm 24.03.18The Pathology Devices Market size is estimated at USD 6.18 billion in 2024, and is expected to reach USD 8.32 billion by 2029, growing at a CAGR of 6.12% during the forecast period (2024-2029).
Key Highlights
- The COVID-19 pandemic had a huge impact on healthcare systems around the world and caused many healthcare facilities to stop providing routine care and pathological testing, putting patients with chronic conditions, including cancer and cardiovascular disorders, at severe risk. For instance, according to an article published in International Medical Journal in January 2022, it was observed that the ratio of the number of pathology tests pre-lockdown and post-lockdown versus baseline period decreased from 1.02 to 0.53, respectively.
- However, the increased coronavirus cases among the population have increased the demand for pathological tests. For instance, as per an article published in Frontiers in Pathology in March 2022, it was found that the number of molecular microbiology tests was more than three times higher during the lockdown period. This has increased the company's focus on developing innovative pathological test kits and devices. Thus, the studied market is expected to grow and regain its full potential in the coming years.
- The increasing prevalence and burden of Alzheimer's disease, autoimmune diseases, cancer, and others around the world are driving the need for effective and advanced diagnostic tests, hence propelling the market growth. For instance, according to the 2022 statistics published by Alzheimer's Association, an estimated 6.5 million Americans age 65 and older were living with Alzheimer's dementia in 2022, and this number is projected to reach 13 million by 2050. Thus, the expected increase in the number of people suffering from Alzheimer's disease is anticipated to fuel the demand for pathology devices that helps physicians in early identifying the disease-causing dementia symptoms, thereby propelling the market growth.
- Additionally, as per the 2023 statistics published by ACS, about 24,810 malignant tumors of the brain or spinal cord (14,280 in males and 10,530 in females) are expected to be diagnosed in the United States in 2023. Thus, the high burden of brain or spinal cord tumors is anticipated to fuel the demand for radionuclide bone scintigraphy (bone scan) for detecting spinal pathologies, hence bolstering the market growth. Moreover, the rising company focus on developing technologically advanced pathological kits and devices is also contributing to the market growth. For instance, in April 2022, Sysmex Europe launched a new three-part differential system, XQ-320 XQ-Series Automated Hematology Analyzer, a robust device that brings excellence in quality to a diverse range of clinical laboratory environments with reliable technology and a new level of usability.
- Therefore, owing to the factors such as the high burden of chronic and infectious diseases and new product launches, the studied market is expected to grow over the forecast period. However, the high cost of devices and stringent regulations, as well as the lack of skilled professionals, are likely to impede the growth of pathological devices over the forecast period.
Pathology Devices Market Trends
Molecular Diagnostics Segment is Expected to Hold Significant Market Share Over the Forecast Period
- Molecular diagnostic devices are used to analyze biological markers in the genome and proteome to detect pathogens or mutations. Based on the technology, molecular diagnostic devices can be segmented into chips and microarrays, mass spectroscopy, next-generation sequencing (NGS), polymerase chain reaction (PCR)-based methods, cytogenetics, and molecular imaging. Factors such as large outbreaks of bacterial and viral epidemics, increasing demand for point-of-care diagnostics, and rapidly evolving technology are driving the growth of the molecular diagnostics segment.
- The growing burden of cancer, cardiovascular diseases, brain tumors, and others is the key factor driving the segment's growth. For instance, according to the statistics published by the British Heart Foundation, in January 2022, more than 7.6 million people were living with cardiovascular diseases in the United Kingdom in 2021.
- Also, as per 2022 statistics published by the National Brain Tumor Society, approximately 88,970 people were diagnosed with a primary brain tumor in the United States in 2022. Thus, the high prevalence of heart diseases and brain tumors among the population is anticipated to propel the demand for molecular diagnosis, which improves diagnosis accuracy, allows for predictive prognosis, and enables target identification. This is expected to fuel the segment growth over the forecast period.
- Furthermore, the increasing company focus on developing technologically advanced products in molecular diagnostics and rising new product launches contributes to market growth. For instance, in March 2022, Mylab Discovery Solutions launched an entire range of routine diagnostic kits and devices, for immunology, biochemistry, and hematology, in conventional and point-of-care formats. This product line expansion assists the labs in using the company's devices and reagent kits to do all routine tests such as liver panels, cardiac profiles, urine panels, hormone panels, fever panels, kidney function tests, cancer markers, and other tests.
- Therefore, owing to the factors such as the high burden of heart diseases and brain tumors, growing demand for precision medicine as well as rising product launches, the studied segment is expected to grow over the forecast period.
North America is Expected to Have the Significant Market Share Over the Forecast Period
- North America is expected to hold a significant market share in the pathology devices market, owing to the rising prevalence of infectious and chronic diseases, better healthcare infrastructure, and the technological advancements of pathological devices.
- The increasing number of chronic and infectious diseases in the region increases the demand for early detection and diagnosis of the condition, which in turn is anticipated to fuel the demand for pathological testing, thereby boosting the market growth. For instance, according to 2023 statistics published by ACS, about 1,958,310 new cancer cases are projected to be diagnosed in the United States in 2023.
- In addition, from the same source, 1,63,020 new cases of colorectal cancer, followed by 2,38,340 cases of lung and bronchus, 297,790 breast cancer, and 288,300 prostate cancer, are expected to be diagnosed in the United States in 2023. Also, as per 2022 data published by CDC, about 12.1 million people in the United States are expected to have atrial fibrillation in 2030. Thus, the rising burden of breast cancer and heart disease cases among the population is anticipated to increase the demand for diagnostic tests for the early detection of cancer, thereby propelling market growth.
- Furthermore, the increasing number of new product launches in the region increases the availability of products and test kits, which is also expected to boost market growth over the forecast period. For instance, in June 2021, Thermo Fisher Scientific Inc. launched TaqPath, a new CE-IVD-marked fast PCR combo kit 2.0, to detect active SARS-CoV-2 infection.
- Also, in March 2021, MatMaCorp launched a new handheld device, MY Real-Time Analyzer (MYRTA), which can carry out polymerase chain reaction (PCR) amplification and real-time fluorescent detection. Therefore, due to the aforementioned factors, such as the high burden of cancer and heart disease among the population, increasing company activities, and the growing number of pathological labs in the region, the studied market is expected to grow over the forecast period.
Pathology Devices Industry Overview
The pathology devices market is fragmented as numerous global and local companies are present in the market. The companies are focusing on adopting various business strategies, such as product launches, agreements, and collaborations, to withhold their market position. Some of the key players in the market are Abbott Laboratories, Becton, Dickinson and Company, Ortho-Clinical Diagnostics, F. Hoffmann-La Roche AG, Danaher Corporation, and Bio-Rad Laboratories, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Chronic and Infectious Diseases
- 4.2.2 Technological Advancements in Pathology Devices
- 4.2.3 Increasing Investment in Healthcare Infrastructure in Developing Countries
- 4.3 Market Restraints
- 4.3.1 High Cost of Devices
- 4.3.2 Stringent Regulations and Lack of Skilled Professionals
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Technology
- 5.1.1 Clinical Chemistry
- 5.1.2 Immunoassays Technology
- 5.1.3 Microbiology
- 5.1.4 Molecular Diagnostics
- 5.1.5 Other Technologies
- 5.2 By Application
- 5.2.1 Drug Discovery and Development
- 5.2.2 Disease Diagnostics
- 5.2.3 Forensic Diagnostics
- 5.2.4 Other Applications
- 5.3 By End-User
- 5.3.1 Pharmaceutical Companies
- 5.3.2 Hospitals and Diagnostic Laboratories
- 5.3.3 Other End-Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Becton, Dickinson and Company
- 6.1.3 Bio-Rad Laboratories
- 6.1.4 Beckman Coulter Inc.
- 6.1.5 Definiens
- 6.1.6 Hamamatsu Photonics
- 6.1.7 Mikroscan Technologies
- 6.1.8 Ortho-Clinical Diagnostics
- 6.1.9 F. Hoffmann-La Roche AG
- 6.1.10 Thermo Fisher Scientific
- 6.1.11 Danaher Corporation
- 6.1.12 Siemens Healthineers

















