
|
시장보고서
상품코드
1445516
세계 단순포진바이러스(HSV) 치료 시장 : 시장 점유율 분석, 업계 동향과 통계, 성장 예측(2024-2029년)Herpes Simplex Virus Treatment - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
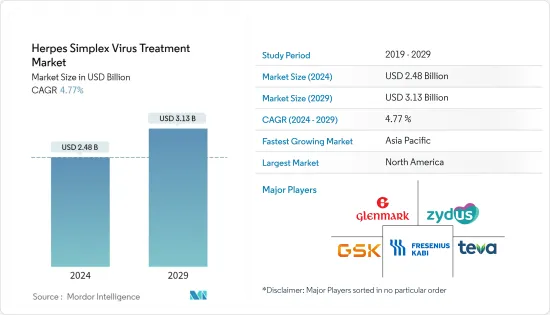
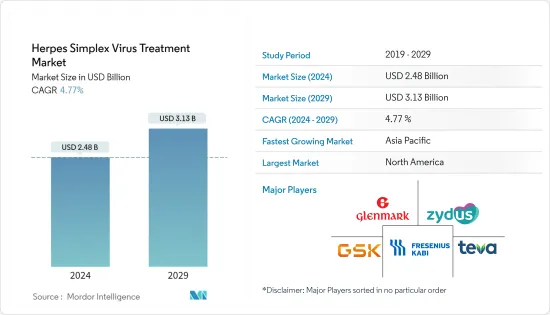
단순포진바이러스(HSV) 치료 시장 규모는 2024년 24억 8,000만 달러로 추정되고, 2029년까지 31억 3,000만 달러에 이를 것으로 예측되고 있으며, 예측 기간(2024년-2029년) 중 복합 연간 성장률(CAGR) 4.77%로 성장할 전망입니다.
COVID-19의 유행은 시장 성장에 큰 영향을 미쳤습니다. 처음에는 COVID-19의 유행에 따라 COVID-19 이외의 병리에 대한 치료법 및 약물 조사 활동이 중단되었습니다. 이는 세계 치료 절차와 의약품 공급망에 영향을 주었고 단순 포진 바이러스 치료 시장에도 영향을 미쳤습니다. 그러나 단순포진바이러스와 SARS-CoV-2 바이러스의 연관성은 유행의 후기 단계에서 연구자들의 많은 연구에서 확립되었습니다. 예를 들어, 2021년 7월에 아일랜드 의학 저널에 게재된 조사 결과에 의하면, 단순포진바이러스 1형 및 수두대상포진 바이러스는 COVID-19와 강하게 관련되어 있어, 단순포진바이러스 1형의 만연은 연구 대상인 COVID-19 그룹에서 1건의 발생률은 2.81%였지만, 병원 인구에서는 0.77%였습니다. 따라서 유행 중에 단순포진바이러스 치료에 대한 수요가 증가했습니다. 이 수요는 치료용 항바이러스제 수요가 증가하기 때문에 시장에 긍정적인 영향을 미치고 시장은 예측 기간에 걸쳐 성장 추세가 지속될 것으로 예상됩니다.
생식기 헤르페스를 포함한 단순포진바이러스 감염의 부담 증가, 연구 개발 활동 증가 등이 시장의 성장 요인으로 생각됩니다.
생식기 헤르페스, HSV 1형 및 HSV 2형과 같은 단순포진바이러스(HSV) 감염의 부담이 증가함에 따라 HSV 약물 및 치료제에 대한 수요가 급속히 증가하고 있습니다. 이것은 주로 예측 기간 동안 단순포진바이러스 치료 시장의 성장을 가속할 것으로 예상됩니다. 예를 들어 2022년 3월 WHO가 갱신한 데이터에 따르면 50세 미만의 약 37억명(총인구의 67%)이 HSV-1에 감염되어 15-49세의 약 4억 9,100만 사람(13%)이 HSV에 감염되었습니다.
2021년 6월 NCBI에 발표된 조사 결과에 따르면 아시아(동남아시아 및 서태평양의 WHO 지역 포함)에서는 매년 약 10명 중 1명이 HSV 2형 감염에 감염된 것으로 나타났습니다. 하고 있습니다. 2는 아시아에서 생식기 궤양 질환(GUD) 사례의 거의 절반, 생식기 헤르페스 사례의 4 분의 3을 차지합니다. 이러한 이유로 HSV 2형 백신과 성 및 생식에 대한 보건 서비스에 대한 보편적인 접근이 필요하며 예측 기간 동안 단순포진바이러스 치료 시장의 성장을 가속할 것으로 예상됩니다.
R&D 활동 증가도 시장 성장에 기여하고 있습니다. HSV 감염은 세계 의료 시스템에 큰 부담을 주므로 HSV 치료 시장에는 높은 잠재력이 있습니다. 이것은 HSV 감염의 효과적인 치료제의 연구와 개발에 대규모 투자로 이어지고 예측 기간 동안 시장 성장을 가속할 것으로 예상됩니다. 예를 들어, 2021년 2월, 연방교육조사부(BMBF)는 프리드리히 알렉산더 대학 Universitatsklinkum Erlangen 임상분자 바이러스학 연구소의 플로리안 풀 박사에게 약 234만 유로(USD 2.49)의 자금을 제공했습니다.(FAU) 헤르페스 바이러스에 대한 신약의 개발에 기여. 자금 제공은 향후 5년간에 걸칩니다. 연구 결과는 시장에 긍정적인 영향을 미치고 성장을 가속할 수 있습니다.
따라서 대상 집단에서 HSV 감염의 부담이 증가하고 새로운 조사에 대한 투자가 증가함에 따라 시장은 예측 기간 동안 크게 성장할 것으로 예상됩니다. 그러나 성병과 관련된 사회적 편견은 제품 리콜 증가와 함께 시장 성장을 방해하는 주요 요인 중 하나입니다.
단순포진바이러스 치료 시장 동향
아시클로비르 부문은 단순포진바이러스 치료 시장에서 중요한 시장 점유율을 유지할 것으로 예상된다.
조빌락스/시타빅으로 판매되는 아시클로비르는 헤르페스의 치료에 사용되는 가장 바람직한 항바이러스제 중 하나이며, 주로 구강 헤르페스 및 단순 헤르페스 뇌염(HSE)에 사용됩니다. 이것은 바이러스 DNA에 들어가 아시클로비르 삼인산으로 전환하여 복제 과정을 제한하는 푸린 뉴클레오시드 유사체입니다.
2022년 4월에 큐루스 저널에 게재된 조사 연구에 따르면, 아시클로비르는 HSV-1 뇌염 환자의 사망률 저하에 비달라빈보다 우수한 효능을 보였으며, 아시클로비르로 치료받은 연구 대상 집단 중 합병증 없이 생존한 환자는 거의 없었습니다. 따라서, 아시클로비르의 이러한 장점은 바람직한 결과를 달성하기 위해 아시클로비르 약물의 채용 증가로 이어지고, 더 높은 채용으로 이어질 것입니다.
이 분야에서 진행 중인 연구개발과 시장관계자에 의한 이 부문의 신약의 발매는 예측기간 동안 아시클로비르 부문의 성장이 더욱 확대될 것으로 예상됩니다. 예를 들어, 2022년 10월에 캠버 퍼머슈티컬즈는 아시클로비르 경구 현탁액을 출시하여 현재 포트폴리오를 확장했습니다. 아시클로비르 경구 현탁액은 헤르페스 바이러스에 대해 활성인 합성 뉴클레오시드 유사체입니다. Camber의 아시클로비르 경구 현탁액은 473 mL 병에서 200 mg/5 mL의 농도로 제공됩니다. 이러한 발전은 예측기간 동안 이 부문의 성장을 가속할 것으로 예상됩니다. 따라서, 이러한 요인들에 의해 아시클로비르 의약 부문은 예측 기간 동안 단순포진바이러스 치료 시장에서 성장할 것으로 예상됩니다.
북미는 시장에서 큰 점유율을 차지하고 있으며 예측 기간 동안에도 마찬가지로 성장할 것으로 예상됩니다.
북미는 지역 전체에서 단순포진바이러스 감염이 만연하기 때문에 HSV 치료 시장에서 큰 점유율을 잡을 것으로 예상됩니다.
2021년 4월 오픈 포럼 감염증 저널에 게재된 연구에 따르면 이 유행은 1세기 동안 크게 진화하여 미국의 15-34세의 개인에게 최대의 영향을 주고 있습니다. 또한 HSV의 높은 발생률은 향후 30년간 지속될 것으로 예상되며 매년 600,000명 이상의 신규 감염이 발생합니다.
또한, 2021년 12월 캐나다 정부가 업데이트한 데이터에 따르면, 역사적으로 HSV-2가 생식기 감염의 가장 흔한 원인임에도 불구하고 생식기 HSV-1 감염의 유병 비율은 특히 캐나다 여성에서 크게 증가하고 있습니다. 헤르페스. 따라서 북미 전역의 대상 집단에서 HSV 감염의 발생률이 증가함에 따라 HSV 치료에 대한 수요가 증가하고 있습니다.
제품 혁신을 위한 연구개발과 임상시험이 증가하고 있으며 시장 성장을 이끌고 있습니다. 예를 들어, 2021년 3월, Precision Vaccinations와 GlaxoSmithKline(GSK)은 HSV 백신(GSK4108771A)으로 알려진 단순포진바이러스 2형(HSV-2) 후보의 제한적인 1상 임상시험을 시작했습니다. 따라서 이러한 진보는 예측 기간 동안 이 지역 시장 성장을 가속할 수 있습니다. 그러므로 HSV 감염의 부담 증가와 시장 관계자의 임상시험 증가로 예측기간 동안 북미 HSV 치료시장이 밀려올 것으로 예상됩니다.
단순포진바이러스 치료 산업 개요
주요 기업은 파트너십, 협정, 협업, 신제품 출시, 지리적 확대, 합병, 인수 등 시장에서의 존재감을 높이기 위해 다양한 성장 전략을 채택하고 있습니다. 현재 시장을 독점하는 기업으로는 Zydus Group, Glenmark Pharmaceuticals Inc., Fresenius Kabi, Teva Pharmaceuticals Industries Ltd, GSK PLC 등이 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 증대하는 단순포진바이러스 감염증의 부담
- 연구개발 활동 증가
- 시장 성장 억제요인
- 성감염과 관련된 사회적 편견과 제품 리콜 증가
- Porter's Five Forces 분석
- 공급기업의 협상력
- 소비자의 협상력
- 신규 참가업체의 위협
- 대체 제품의 위협
- 경쟁 기업간 경쟁 관계의 격렬
제5장 시장 세분화
- 의약품별
- 아시클로빌
- 발라시클로빌
- 팜시클로빌
- 기타
- 투여 경로별
- 경구
- 주사
- 국소
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 영국
- 독일
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 세계 기타 지역
- 북미
제6장 경쟁 구도
- 기업 프로파일
- Teva Pharmaceuticals Industries Ltd
- Apotex Inc.
- Viatris Inc.
- Zydus Group
- GlaxoSmithKline PLC
- Carlsbad Tech
- Emcure Pharmaceuticals Ltd(Avet Pharmaceuticals Inc.)
- Glenmark Pharmaceuticals
- Fresenius SE & Co. KGaA
- AiCuris GmbH &Co. KG
- Agenus Inc.
- BlueWillow Biologics
- Novartis AG
제7장 시장 기회와 미래 동향
BJH 24.03.15The Herpes Simplex Virus Treatment Market size is estimated at USD 2.48 billion in 2024, and is expected to reach USD 3.13 billion by 2029, growing at a CAGR of 4.77% during the forecast period (2024-2029).
The COVID-19 pandemic significantly impacted the market's growth. Initially, the COVID-19 pandemic disrupted the research activities of therapies and drugs for medical conditions other than COVID-19. It impacted the treatment procedures and supply chain of pharmaceuticals worldwide, which affected the herpes simplex virus treatment market. However, the association of herpes simplex virus with the SARS-CoV-2 virus was established in many studies by researchers during the later phases of the pandemic. For instance, according to a research study published in the Irish Journal of Medical Science in July 2021, herpes simplex virus type-1 and varicella-zoster viruses are strongly associated with COVID-19 infection, and the prevalence of the herpes simplex virus type-1 occurrence in the studied COVID-19 group was 2.81% compared to 0.77% in the hospital population. Thus, there was a rise in the demand for herpes simplex virus treatment during the pandemic. This demand impacted the market positively as it increased the demand for anti-viral drugs for treatment, and the market is anticipated to continue its growth trend over the forecast period.
Factors such as the growing burden of herpes simplex virus infections, including genital herpes, and increasing R&D activities are factors attributed to the market's growth.
With the increasing burden of herpes simplex virus (HSV) infections, such as genital herpes, HSV type 1, and HSV type 2, the demand for HSV drugs and therapeutics is increasing rapidly. This is primarily expected to drive the growth of the herpes simplex virus treatment market during the forecast period. For instance, as per data updated by WHO in March 2022, nearly 3.7 billion people under age 50 (67% of the total population) had HSV-1 infection, while approximately 491 million people aged 15-49 years (13%) had HSV-2 infection in 2021, globally.
As per a research study published in NCBI in June 2021, in Asia (including the WHO regions of Southeast Asia and the Western Pacific), about 1 in 10 persons were found to be infected with HSV type-2 infection annually, where HSV type-2 accounts for almost half of the genital ulcer disease (GUD) cases and three-quarters of genital herpes cases in Asia. Due to this, there is a need for vaccines for HSV type-2 and universal access to sexual and reproductive health services, which is expected to boost the growth of the herpes simplex virus treatment market during the forecast period.
Increasing R&D activities are also contributing to the market's growth. There is high potential in the HSV treatment market owing to the huge burden of HSV infections on healthcare systems worldwide. This is leading to huge investments in the R&D of effective therapeutics for HSV infections and is expected to boost the market's growth over the forecast period. For instance, in February 2021, the Federal Ministry of Education and Research (BMBF) provided funding of nearly EUR 2.34 million (USD 2.49) to Dr. Florian Full at the Institute of Clinical and Molecular Virology at Universitatsklinkum Erlangen at Friedrich-Alexander-Universitat (FAU) for the development of a new drug against the herpes virus. The funding is for the next five years. The study's outcome may impact the market positively and boost the growth.
Therefore, owing to the increasing burden of HSV infections among the target population and the increase in investments for new research, the market is expected to witness significant growth during the forecast period. However, the social stigma associated with sexually transmitted diseases, coupled with rising product recalls, is one of the major factors impeding the market's growth.
Herpes Simplex Virus Treatment Market Trends
Acyclovir Segment is Expected to Hold a Significant Market Share in the Herpes Simplex Virus Treatment Market
Acyclovir, marketed as Zovirax/Sitavig, is one of the most preferred anti-viral agents used for the treatment of herpes, which is primarily used against oral herpes and herpes simplex encephalitis (HSE). It is a purine nucleoside analog that enters viral DNA to restrict its replication process by converting it into acyclovir triphosphate.
According to the research study published in the Cureus journal in April 2022, acyclovir showed superior efficacy than vidarabine in reducing mortality in patients with HSV-1 encephalitis, and few patients among the studied population treated with acyclovir survived without any comorbidities. Thus, such benefits of acyclovir will lead to increased adoption of acyclovir medications to achieve desired results, leading to higher adoption.
The ongoing R&D in the area and the launch of new drugs in the segment by market players are further expected to augment the growth of the acyclovir segment over the forecast period. For instance, in October 2022, Camber Pharmaceuticals launched Acyclovir Oral Suspension and extended its current portfolio. Acyclovir Oral Suspension is a synthetic nucleoside analog active against herpes viruses. Acyclovir Oral Suspension from Camber is available in 200 mg/5 mL strength in 473 mL bottles. Such developments are expected to fuel the segment's growth over the forecast period. Thus, due to such factors, the acyclovir drug segment is expected to grow in the herpes simplex virus treatment market during the forecast period.
North America Holds a Significant Share in the Market and Expected to do Same Over the Forecast Period
North America is expected to hold a significant share of the HSV treatment market owing to the growing prevalence of infections of the herpes simplex virus across the region.
According to the study published in Open Forum Infectious Diseases Journal in April 2021, the epidemic has evolved significantly over the course of a century, having the biggest impact on individuals aged 15-34 years in the United States. In addition, the high incidence of HSV is expected to continue for the following three decades, with more than 600,000 new infections occurring every year.
In addition, as per the data updated by the Government of Canada in December 2021, the prevalence of genital HSV-1 infections has significantly increased, especially in females in Canada, even though historically, HSV-2 has been the most common cause of genital herpes. Thus, with the rising incidence of HSV infections among the target population across North America, the demand for HSV treatment is increasing.
The R&D and clinical trials for product innovation are rising and driving the market's growth. For instance, in March 2021, Precision Vaccinations and GlaxoSmithKline (GSK) launched a limited Phase 1 clinical trial for a herpes simplex virus type 2 (HSV-2) candidate known as the HSV vaccine (GSK4108771A). Thus, such advancements may boost the region's market growth during the forecast period. Hence, the rising burden of HSV infections and rising clinical trials by market players are expected to boost the HSV treatment market in North America over the forecast period.
Herpes Simplex Virus Treatment Industry Overview
The key players are adopting different growth strategies to enhance their market presence, such as partnerships, agreements, collaborations, new product launches, geographical expansions, mergers, and acquisitions. Some companies currently dominating the market are Zydus Group, Glenmark Pharmaceuticals Inc., Fresenius Kabi, Teva Pharmaceuticals Industries Ltd, and GSK PLC.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden of Herpes Simplex Virus Infections
- 4.2.2 Increasing R&D Activities
- 4.3 Market Restraints
- 4.3.1 Social Stigma Associated with Sexually Transmitted Diseases, Coupled with Rising Product Recalls
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Drug
- 5.1.1 Acyclovir
- 5.1.2 Valacyclovir
- 5.1.3 Famciclovir
- 5.1.4 Other Drugs
- 5.2 By Route of Administration
- 5.2.1 Oral
- 5.2.2 Injection
- 5.2.3 Topical
- 5.3 By Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 United Kingdom
- 5.3.2.2 Germany
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Rest of the World
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Teva Pharmaceuticals Industries Ltd
- 6.1.2 Apotex Inc.
- 6.1.3 Viatris Inc.
- 6.1.4 Zydus Group
- 6.1.5 GlaxoSmithKline PLC
- 6.1.6 Carlsbad Tech
- 6.1.7 Emcure Pharmaceuticals Ltd (Avet Pharmaceuticals Inc.)
- 6.1.8 Glenmark Pharmaceuticals
- 6.1.9 Fresenius SE & Co. KGaA
- 6.1.10 AiCuris GmbH & Co. KG
- 6.1.11 Agenus Inc.
- 6.1.12 BlueWillow Biologics
- 6.1.13 Novartis AG















