
|
시장보고서
상품코드
1521344
인공심장 시장 : 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Global Artificial Heart - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
세계의 인공심장 시장 규모는 2024년에 26억 달러로 추정되고, 2029년에는 53억 달러에 이를 것으로 예측되며, 예측 기간(2024-2029년)의 CAGR은 15.40%로 성장할 전망입니다.

심혈관 질환의 부담 증가와 기술 진보가 인공심장 시장의 성장을 가속하는 주요 요인입니다. 노인 인구 증가와 앉기 쉬운 라이프 스타일은 심혈관 질환의 위험을 높입니다. 연구개발 활동의 활성화도 시장을 끌어올릴 것으로 예상됩니다.
주요 하이라이트
- 영국 심장재단(BHF)이 2024년 1월 발표한 사실 시트에 따르면 영국에서는 약 760만 명이 심장병을 앓고 있습니다. 영국에서는 매년 약 10만 명의 심장병 환자가 입원하고 있습니다.
- 현재 세계 약 6억 2,000만 명이 심장병과 순환기 질환을 앓고 있습니다. 심장병의 유병률은 아시아에서 가장 높았으며 유럽, 중동, 터키, 아프리카가 되었습니다.
- 심혈관 질환의 부담이 증가함에 따라 심부전에 따른 사망률을 저하시키는 혁신적인 기술에 대한 수요가 높아지고 있어 인공심장 수요가 증가하고 있습니다.
- 세계의 모든 지역과 지역에서 인구의 고령화가 진행되고 있으며, 향후 수십 년에 걸쳐 고령화가 진행될 것으로 예측되고 있습니다. 예를 들어 유엔의 '세계인구고령화 2023'에 따르면 선진국들은 인구고령화가 보다 앞선 단계로 이행하고 노인 비율은 2023년 20%에서 2050년 28%로 상승할 것으로 예측됩니다. 기타 개발도상국에서도 이 비율은 9%에서 17%로 상승하고, 후발 개발도상국에서는 2050년까지 4%에서 6% 이상으로 상승할 것으로 예상됩니다. 이러한 고령화 인구 증가는 심혈관 질환의 높은 위험을 초래하고 시장 성장을 가속합니다.
- 따라서 심장질환의 유병률 상승과 조사 건수의 급증으로 시장은 향후 수년간 크게 성장할 것으로 예상됩니다. 그러나 인공심장과 관련 수술 비용이 높으면 예측 기간 동안 시장 성장을 방해할 수 있습니다.
인공심장 시장 동향
심실 보조 장치가 큰 점유율을 차지할 전망
- 보조 인공심장(VAD)은 심장 아래의 방(심실)에서 몸의 나머지 부분으로 혈액을 보낼 수 있습니다. 보조 인공심장은 심장이 약한 환자와 심부전 환자에게 필수적입니다. 심장이 회복될 때까지 또는 심장 이식 전까지 일시적으로 심장 기능을 유지합니다. 일부 만성 심장 질환은 전신에 혈액을 보내기 위해 심장에 수술로 이식되는 기계적 펌프이기 때문에 지속적인 보조 요법으로 사용됩니다.
- 제품의 신흥국 시장의 발전, 연구개발 활동의 활성화, 주요 시장 진출기업의 전략 등이 시장의 성장을 가속할 것으로 예상됩니다. 예를 들면
- 2022년 7월, 중국 국가 의료 제품 관리국은 심각한 심부전으로 인해 국산 개발된 인공심장 판매 승인을 발행했습니다. 이것은 강자성 유체 구동의 임베디드 좌심실 보조 장치입니다.
- 2022년 3월, 펜실베니아 주립 헬스 밀턴 S 허시 메디컬 센터는 새로 설계된 EvaHeart2 좌심실 보조 시스템(LVAS)을 중증 심부전 환자에 이식한 미국에서 두 번째 병원이 되었습니다. 이 수술은 2022년까지 40명의 임상시설과 399명의 중증 심부전 환자를 포함하여 EvaHeart2를 평가하기 위한 다시설 임상시험인 COMPETENCE Trial의 일부였습니다.
- 게다가, 유명한 기업은 첨단 보조 인공심장 개발에 적극적으로 참여하고 있으며 예측 기간 동안이 부문의 성장을 가속할 것으로 예상됩니다.
- 예를 들어, 2023년 6월, Magenta Medical은 고위험 경피적 관상동맥 인터벤션(HR-PCI)을 위한 고가식 경피적 좌심실 보조 장치(VAD)로 알려진 최소 심장 펌프의 임상시험 연구를 시작했습니다. 이 조사의 목적은 심장 수술 중 버스큘러 액세스 합병증을 줄이는 것입니다.
- 이와 같이 이 조사 부문은 제품 조사 증가, 연구 개발의 활성화, 주요 시장 진출기업이 채용하는 전략에 의해 큰 성장에 기여할 것으로 기대되고 있습니다.
북미가 시장에서 큰 점유율을 차지할 전망
- 인공심장 시장의 지역 분석에 따르면 북미는 세계 시장에서 큰 점유율을 차지합니다. 북미 인공심장 시장 성장의 주요 원동력은 심혈관 질환 부담 증가, 기술 채용 증가, 제품 승인 증가, 투자 증가, 주요 시장 진출기업의 주요 이니셔티브입니다.
- 심부전으로 이어지는 심혈관 질환의 부담 증가는 이 지역의 인공심장 시장의 진전을 뒷받침할 것으로 기대됩니다. 예를 들어, 2023년 5월 CDC가 발표한 기사에 따르면 매년 약 65만 9,000명의 미국인이 심장 발작을 일으키고 있습니다. 따라서 이 지역에서는 심장마비의 유병률이 높기 때문에 심장의 효율을 높일 수 있는 장비의 지속적인 요구가 있습니다.
- 이 지역의 제품 및 서비스 출시 증가도 시장을 크게 견인할 것으로 예상됩니다. 예를 들어, 2022년 2월, Carmat는 미국에서 인공심장의 새로운 버전의 FDA 승인을 받았습니다. 이 새로운 버전은 PIVOTAL 시험에서 얻은 임상 경험별 인공심장과 착용 가능한 시스템의 개선을 포함합니다.
- 심장의 기능을 지원하는 기술을 개발하기 위한 연구개발 활동이 활발해지고 있는 것도 성장을 뒷받침하는 큰 요인이 되고 있습니다. 예를 들어, 2023년 2월 하버드 대학의 연구팀은 인간의 심장 세포 수축을 이용하여 자율적으로 수영하는 물고기의 무리를 설계했습니다. 이 실험은 심박 조율기 기술을 발전시키고 인간을 위한 인공심장의 개발을 개선할 수 있습니다.
- 따라서 심장병의 유병률이 높고 유명한 진입기업에 의한 제품 출시가 많기 때문에 조사된 시장은 북미에서 유리한 성장을 보일 것으로 기대되고 있습니다.
인공심장 산업 개요
세계의 인공심장 시장은 반 고정적입니다. 합병, 인수, 조사 활동, 제휴 및 기타 중요한 전략의 채택이 시장 성장을 뒷받침할 것으로 기대됩니다. 이 시장의 주요 기업으로는 SynCardia Systems LLC, CryoLife Inc., Abiomed, Carmat, Abbott Laboratories 등이 있습니다.
기타 혜택
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 심혈관질환의 부담증
- 기술의 진보
- 시장 성장 억제요인
- 인공심장과 관련 수술의 높은 비용
- Porter's Five Forces 분석
- 신규 진입업자의 위협
- 구매자 및 소비자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계의 강도
제5장 시장 세분화(시장 규모(단위 : 달러))
- 유형별
- 보조 인공심장
- 전인공심장
- 인공 심폐
- 기타
- 전원별
- 내장 배터리
- 외부 배터리
- 최종 사용자별
- 병원 및 클리닉
- 전문 클리닉
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC 국가
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- SynCardia Systems LLC
- CryoLife Inc.
- Abiomed
- Carmat
- Abbott
- BiVACOR Inc.
- ReinHeart TAH GmbH
- Jarvik Heart Inc.
- Calon Cardio-Technology Ltd
- Berlin Heart Inc.
제7장 시장 기회 및 향후 동향
AJY 24.08.02The Global Artificial Heart Market size is estimated at USD 2.60 billion in 2024, and is expected to reach USD 5.30 billion by 2029, growing at a CAGR of 15.40% during the forecast period (2024-2029).
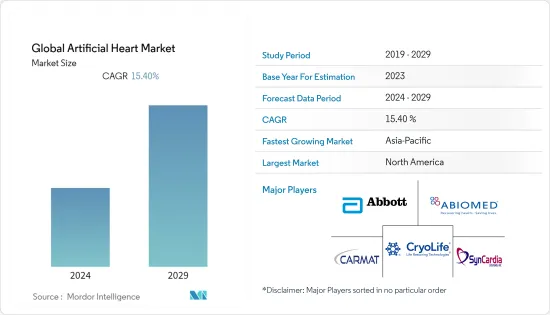
The growing burden of cardiovascular diseases and technological advancements are the major factors driving the growth of the artificial heart market. The growth of the geriatric population and sedentary lifestyles are increasing the risk of cardiovascular diseases. Rising research and development activities are also expected to boost the market.
Key Highlights
- According to the factsheet published by the British Heart Foundation (BHF) in January 2024, approximately 7.6 million people in the United Kingdom were suffering from heart disease. Approximately 100,000 patients suffering from heart disease are admitted to hospitals in the United Kingdom every year.
- Around 620 million people are currently living with heart and circulatory diseases worldwide. The prevalence of heart disease is highest in Asia, followed by Europe and the Middle East, Turkey, and Africa.
- Due to the rising burden of cardiovascular diseases, there is an increasing demand for innovative technologies that can decrease the mortality rates associated with heart failure, thereby increasing the demand for artificial hearts.
- All regions and areas worldwide are experiencing population aging, and it is projected to continue to do so over the next several decades. For instance, according to the United Nations World Population Ageing 2023, developed countries are expected to move to a more advanced stage of population aging, with the proportion of older persons rising from 20% in 2023 to 28% in 2050. For other developing countries, this proportion also rises from 9 to 17%, while for least developed countries, it is expected to rise from 4% to over 6% by 2050. Such an increase in the aging population poses a high risk for cardiovascular diseases and thus drives the growth of the market.
- Therefore, owing to the rising prevalence of heart diseases and the surging number of research studies, the market is expected to grow significantly over the coming years. However, the high cost of artificial hearts and related surgeries may hinder the market's growth during the forecast period.
Artificial Heart Market Trends
Ventricular Assist Devices are Expected to Account for a Large Share
- A ventricular assist device (VAD) helps pump blood from the lower chambers of the heart (ventricles) to the rest of the body. VAD is essential for patients with weakened hearts or heart failures. They temporarily maintain the heart function until the heart recovers or before the heart transplant. In some chronic heart conditions, they are used as permanent support therapy, as they are mechanical pumps surgically implanted in the heart to pump blood into the whole body.
- Factors such as increasing product launches, rising research and development activities, and strategies adopted by key market players will likely drive the growth of the market. For instance,
- In July 2022, China's National Medical Products Administration issued marketing approval for a domestically developed artificial heart for severe heart failure. It is an implantable ferrofluids-driven left ventricular assist device.
- In March 2022, Penn State Health Milton S. Hershey Medical Center became the second hospital in the United States to implant a newly designed EvaHeart2 Left Ventricular Assist System (LVAS) in a patient with severe heart failure. The procedure was part of the COMPETENCE Trial, a multi-center clinical study to evaluate EvaHeart2, including 40 clinical sites and 399 patients with severe heart failure through 2022.
- Furthermore, the prominent players are actively participating in the development of advanced VAD, which is projected to drive the growth of the segment during the forecast period.
- For instance, in June 2023, Magenta Medical commenced a clinical trial study for its smallest heart pump, known as elevated percutaneous Left Ventricular Assist Device (VAD), for high-risk percutaneous coronary intervention (HR-PCI). The aim of the study is to reduce vascular access complications during heart surgery.
- Thus, the studied segment is expected to contribute to significant growth due to the increasing product launches, rising research and development activities, and strategies adopted by key market players.
North America is Expected to Hold a Significant Share in the Market
- The geographical analysis of the artificial heart market shows that North America holds a significant market share in the global market. The primary driving factors for the growth of the North American artificial heart market are the growing burden of cardiovascular diseases, rising adoption of technologies, increasing product approvals, increasing investments, and key initiatives taken by the key market players.
- The rising burden of cardiovascular diseases leading to heart failure is expected to boost the advancements in the artificial heart market in the region. For instance, according to the article published by the CDC in May 2023, about 659,000 Americans have a heart attack every year. Hence, due to the high prevalence of heart attacks in the region, there is a continuous need for devices that can increase the efficiency of hearts.
- The increase in the launch of products and services in the region is also anticipated to drive the market significantly. For instance, in February 2022, Carmat received FDA approval for its new version of its artificial heart in the United States. This new device version includes improvements in the prosthesis and the wearable system based on clinical experience gained in the PIVOTAL study.
- The rising research and development activities for developing technologies supporting heart functioning are another major factor likely to drive growth. For instance, in February 2023, Researchers from Harvard University engineered a school of fish that uses the contractions of human heart cells to swim autonomously. This experiment could advance pacemaker technology and improve the development of artificial hearts for humans.
- Therefore, due to the high prevalence of heart diseases and the number of product launches by prominent players, the market studied is expected to witness lucrative growth in North America.
Artificial Heart Industry Overview
The global artificial heart market is semi-consolidated. The adoption of key strategies such as mergers, acquisitions, research activities, partnerships, and others is expected to boost the growth of the market. Major players in the market include SynCardia Systems LLC, CryoLife Inc., Abiomed, Carmat, and Abbott Laboratories, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden of Cardiovascular Diseases
- 4.2.2 Technological Advancements
- 4.3 Market Restraints
- 4.3.1 High Cost of Artificial Heart and Related Surgery
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - in USD)
- 5.1 By Type
- 5.1.1 Ventricular Assist Device
- 5.1.2 Total Artificial Heart
- 5.1.3 Heart-Lung Machine
- 5.1.4 Other Types
- 5.2 By Power Source
- 5.2.1 Internal Battery
- 5.2.2 External Battery
- 5.3 By End User
- 5.3.1 Hospitals and Clinics
- 5.3.2 Specialty Clinics
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 SynCardia Systems LLC
- 6.1.2 CryoLife Inc.
- 6.1.3 Abiomed
- 6.1.4 Carmat
- 6.1.5 Abbott
- 6.1.6 BiVACOR Inc.
- 6.1.7 ReinHeart TAH GmbH
- 6.1.8 Jarvik Heart Inc.
- 6.1.9 Calon Cardio-Technology Ltd
- 6.1.10 Berlin Heart Inc.

















