
|
시장보고서
상품코드
1521694
뉴로테크놀러지 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Neurotechnology - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
뉴로테크놀러지 시장 규모는 2024년 151억 8,000만 달러로 추정되며, 2029년에는 285억 7,000만 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 13.5%의 CAGR을 기록할 것으로 예상됩니다.
신경전자 시장의 성장을 촉진하는 요인으로는 신경 질환의 유병률 증가, 신경 과학 및 기술의 급속한 발전, 개선된 치료 옵션에 대한 수요 증가 등을 들 수 있습니다.
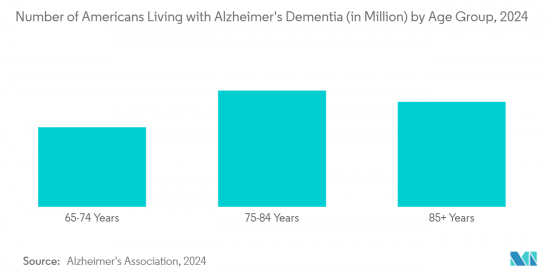
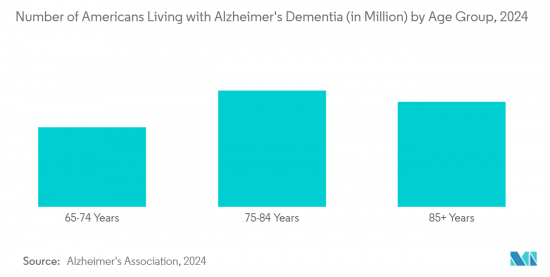
알츠하이머병, 파킨슨병, 간질, 뇌졸중과 같은 신경 질환의 발병률이 증가함에 따라 효과적인 진단 및 치료 솔루션에 대한 수요가 증가하고 있습니다. Alzheimer's Association 2024, Alzheimer's Disease Facts and Figures에 발표된 Alzheimer's Disease Facts and Figures에 따르면, 미국의 65세 이상 인구는 2021년 2021년 5,800만 명에서 2050년에는 8,800만 명으로 증가할 것으로 예측됩니다. 이 자료에 따르면, 2024년 말까지 65세 이상 미국인 중 약 650만 명이 알츠하이머병을 앓기 시작할 것이며, 2060년 말까지 이 숫자는 1,400만 명에 달할 것으로 예측됩니다.
국립 신경질환 및 뇌졸중 연구소가 2024년 1월 발표한 자료에 따르면, 파킨슨병(PD)은 미국에서 두 번째로 흔한 신경퇴행성 질환으로, 매년 미국 노인 인구의 약 5-10%가 앓고 있습니다. 자료에 따르면 매년 약 50만 명이 파킨슨병 진단을 받고 있습니다.
세계보건기구(WHO)가 2024년 2월 발표한 자료에 따르면, 전 세계적으로 5,000만 명 이상이 간질을 앓고 있으며, 가장 흔한 신경질환 중 하나입니다. 간질 환자의 약 80%가 중저소득 국가에 거주하고 있습니다. 적절한 진단과 치료를 받으면 간질 환자의 약 70%가 발작 없이 생활할 수 있는 것으로 추정됩니다. 이처럼 알츠하이머병, 파킨슨병, 간질 환자의 증가는 조기 발견, 정확한 진단 및 이들 질환의 표적 치료를 위한 새로운 가능성을 제공하는 뉴로테크놀러지 제품에 대한 수요를 촉진할 것으로 예상됩니다.
또한, 제품 출시 및 승인 건수의 증가는 예측 기간 동안 시장 성장을 촉진할 것으로 보입니다. 예를 들어, 2023년 5월, Abbott는 허리 수술을 받을 수 없는 사람들의 만성 요통을 치료하기 위해 척추 자극(SCS) 시스템에 대한 FDA 승인을 획득했습니다. 이런 종류의 요통은 비수술적 요통으로 알려져 있습니다. 이 새로운 적응증은 Eterna SCS 플랫폼과 Proclaim SCS 제품군을 포함한 Abbott의 미국 내 SCS에 적용되며, 2023년 1월에는 세계 의료 기술 기업인 Axonix가 4세대 충전식 천골 신경 조절 시스템에 대해 미국 식품의약국(FDA)의 승인을 받았습니다. 미국 식품의약국(FDA)의 승인을 받았습니다. 이러한 제품 승인 건수의 증가는 혁신적인 제품의 가용성을 높이고 제품의 안전성과 효과에 대한 사람들의 신뢰를 높임으로써 시장 성장을 촉진할 것으로 예상됩니다.
그러나 높은 시장 개척 비용과 복잡한 규제 환경은 예측 기간 동안 시장 성장을 저해하는 요인으로 작용할 수 있습니다.
뉴로테크놀러지 시장 동향
신경자극기 부문은 예측 기간 동안 큰 폭의 성장이 예상
신경 자극기는 신경 활동을 조절하기 위해 신경계의 특정 신경 또는 부위에 전기적 또는 자기적 자극을 가하는 것으로 통증 관리, 신경 질환 치료, 신경 조절, 재활 등 다양한 치료 목적으로 사용할 수 있습니다. 이러한 장치는 이식하거나 외부 장치로 사용할 수 있습니다. 만성 통증, 운동 장애, 정신 질환 및 기타 신경 질환을 앓고 있는 환자들에게 맞춤형 치료를 제공하도록 설계되었습니다.
이 시장 부문의 성장을 이끄는 주요 요인으로는 기술의 급속한 발전, 비침습적 및 최소침습적 치료에 대한 환자 선호도, 제품 출시 수 증가 등을 들 수 있습니다. 신경자극기는 기존의 신경 질환뿐만 아니라 보다 광범위한 적응증에 사용되고 있습니다. 현재는 통증 관리, 비만 치료, 중독 치료, 우울증, 불안장애 등 정신질환에 대한 적용이 검토되고 있으며, 이로 인해 대응 가능한 시장이 확대되어 빠른 성장에 기여하고 있습니다.
American Academy of Neurology Journal이 2022년 1월에 발표한 논문에 따르면, 신경 자극기는 운동 장애, 간질, 통증, 우울증 치료에 대한 승인을 받고 있습니다. 이 소식통에 따르면 2035년까지 신경 해부학 네트워크에 대한 이해, 자극의 작용 메커니즘, 재료 과학의 범위 확대, 소형화, 에너지 저장 시설 및 더 나은 전달의 발전으로 인해 신경 자극기의 사용이 증가하여 예측 기간 동안이 시장 부문의 성장을 크게 촉진할 것이라고합니다.
또한, 제품 출시 및 제휴와 같은 주요 기업의 전략적 활동 증가와 규제 당국의 제품 승인 급증은 시장 성장을 촉진할 것으로 예상되며, 2022년 1월 TensCare는 Arab Health 2022에서 최신 경피적 전기신경자극기를 전시했습니다. 이 치료기는 당뇨병성 신경병증, 요통, 좌골신경통, 골관절염, 출산 후 외상 등 만성 통증 질환의 장기적인 치료에서 약물 없는 통증 완화를 위해 널리 사용되고 있습니다.
2022년 11월, 상업화 단계의 바이오 전자 의료 기업 일렉트로코어(Electrocore)는 감마코어 사파이어 비침습적 미주신경 자극기(nVNS)에 대해 벨기에 제약협회로부터 자체 국가 제품 코드 번호를 획득했습니다. 또한, 2022년 8월 Medtronic Private Limited는 인도에서 운동 장애 및 간질과 관련된 증상을 치료하는 뇌심부자극(DBS) 치료용 센사이트(SenSight) 지향성 리드 시스템을 출시하였습니다.
제품 출시 수 증가와 급속한 기술 발전 등의 요인이 예측 기간 동안 시장 성장을 촉진할 것으로 예상됩니다.
북미는 예측 기간 동안 큰 폭의 성장이 예상
북미 시장은 노인 인구 증가, 신경질환 증가, 주요 기업의 전략적 제품 출시 등의 요인으로 인해 건강한 성장세를 보일 가능성이 높습니다.
이 지역에서는 상당수의 사람들이 다양한 신경 질환을 앓고 있어 예측 기간 동안 신경 기술 장치에 대한 높은 수요가 발생할 것으로 예상되며, 2024년 1월 Parkinson's Foundation이 업데이트한 데이터에 따르면, 미국에서는 매년 약 9만 명이 파킨슨병 진단을 받을 것으로 예상됩니다. 파킨슨병 진단을 받고 있으며, 2030년 말까지 미국 전역에서 약 120만 명이 파킨슨병에 걸릴 것으로 예상됩니다.
2024년 1월 캐나다 통계청이 발표한 자료에 따르면, 약 75만 명의 캐나다인이 알츠하이머병 및 기타 치매를 앓고 있으며, 2022년 10월 The Journal of Prevention of Alzheimer's Disease가 발표한 논문에 따르면, 2022년 10월에 약 75만 명의 캐나다인이 알츠하이머병 및 기타 치매 관련 질환을 앓고 있는 것으로 나타났다, 알츠하이머병 및 기타 치매 관련 질환은 멕시코에서 장애 조정 생존년수(DALY)를 초래하는 모든 신경계 질환 중 2위를 차지합니다. 북미의 신경질환 증가는 신경질환의 진단과 치료를 위한 혁신적인 뉴로테크놀러지 개발에 박차를 가할 것으로 예상됩니다. 이러한 신흥국 시장 개척은 북미 시장의 성장을 촉진할 것으로 예상됩니다.
또한, 2024년 1월 Abbott는 원격 프로그래밍이 가능한 최소형 충전식 뇌심부자극(DBS) 장치인 Liberta RC DBS 시스템(원격 프로그래밍이 가능한 최소형 충전식 뇌심부자극(DBS) 장치)의 출시 승인을 FDA로부터 획득하여 운동 장애를 가진 사람들을 치료할 수 있게 되었습니다. FDA로부터 승인을 받았습니다.
네브로는 비수술적 난치성 요통(NSRBP) 치료를 위한 센자 척수 자극(SCS) 시스템의 적응증 확대에 대한 FDA 승인을 받았으며, 2022년 1월에는 메드트로닉이 당뇨병성 말초신경병증(DPN)에 따른 만성 통증 치료를 위한 충전식 신경자극기인 Intellis와 충전식 신경자극기 Vanta에 대한 FDA 승인을 획득했습니다.
이러한 신경 장애 사례의 증가와 제품 개발의 증가 등의 요인이 예측 기간 동안 지역 시장의 성장을 촉진할 것으로 예상됩니다.
뉴로테크놀러지 산업 개요
뉴로테크놀러지 시장은 세계 및 지역적으로 사업을 전개하는 여러 기업이 존재하기 때문에 반독점적 성격을 띠고 있습니다. 주요 기업들은 합병, 인수, 제휴 등 다양한 전략적 활동에 지속적으로 참여하고 있습니다. 경쟁 환경은 시장 점유율과 인지도가 높은 현지 기업 및 지역 기업들이 존재감을 드러내고 있습니다.
기타 혜택:
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 소개
- 조사 가정과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 신경질환 유병률 상승
- 신경과학과 기술의 급속한 진보
- 치료법 개선에 대한 수요 증가
- 시장 성장 억제요인
- 뉴로테크놀러지 디바이스의 고비용
- 엄격한 규제 프레임워크
- Porter's Five Forces 분석
- 신규 참여업체의 위협
- 구매자/소비자의 협상력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업 간의 경쟁 강도
제5장 시장 세분화(시장 규모 - 금액)
- 제품 유형별
- 신경보철
- 신경자극 기기
- 뇌 컴퓨터 인터페이스
- 기타 제품
- 용도별
- 신경변성질환
- 정신신경질환
- 만성 통증 관리
- 기타 용도
- 최종사용자별
- 병원 및 클리닉
- 연구기관 및 학술 센터
- 기타 최종사용자
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 상황
- 기업 개요
- Medtronic
- Abbott Laboratories
- Boston Scientific
- Siemens Healthineers
- GE Healthcare
- LivaNova PLC
- NeuroPace
- Neuronetics
- Koninklijke Philips N.V.
- Elekta AB
제7장 시장 기회와 향후 동향
ksm 24.08.01The Neurotechnology Market size is estimated at USD 15.18 billion in 2024, and is expected to reach USD 28.57 billion by 2029, growing at a CAGR of 13.5% during the forecast period (2024-2029).
The factors that are driving the growth of the neuroelectronics market include the increasing prevalence of neurological disorders, rapid advancements in neuroscience and technology, and the rising demand for improved treatment options.
The rising incidence of neurological disorders, such as Alzheimer's disease, Parkinson's disease, epilepsy, and stroke, has led to a rising demand for effective diagnostic and therapeutic solutions. This situation is projected to drive the growth of the market during the forecast period. According to the Alzheimer's Association 2024, published in Alzheimer's Disease Facts and Figures, the American population aged 65 years and above has been projected to grow from 58 million in 2021 to 88 million by 2050. As per the same source, an estimated 6.5 million Americans aged 65 years and above are expected to start suffering from Alzheimer's disease by the end of 2024, and this number is estimated to reach 14 million by the end of 2060.
According to the data published by the National Institute of Neurological Disorders and Stroke in January 2024, Parkinson's disease (PD) is the second-most common neurodegenerative disorder in the United States, which affects around 5%-10% of the geriatric population of the country every year. As per the same source, approximately 500,000 citizens are diagnosed with Parkinson's disease every year.
According to the data published by the WHO in February 2024, more than 50 million people across the world suffer from epilepsy, making it one of the most common neurological diseases. Around 80% of people with epilepsy live in low- and middle-income countries. Estimates suggest that about 70% of people suffering from epilepsy could live seizure-free if they are properly diagnosed and treated. Thus, the rising number of people suffering from Alzheimer's disease, Parkinson's disease, and epilepsy is projected to drive the demand for neurotechnology products to offer new possibilities for early detection, precise diagnosis, and the targeted treatment of these conditions.
The rising number of product launches and approvals is also likely to drive the growth of the market during the forecast period. For instance, in May 2023, Abbott received the FDA's approval for its spinal cord stimulation (SCS) systems for treating chronic back pain in people who cannot undergo back surgery. This kind of back pain is known as non-surgical back pain. This new indication applies to Abbott's SCS in the United States, including the Eterna SCS platform and the Proclaim SCS family. In January 2023, Axonics Inc., a global medical technology company, received the United States Food and Drug Administration's approval for the company's fourth-generation rechargeable sacral neuromodulation system. Thus, the rising number of product approvals is projected to drive the growth of the market by boosting the availability of innovative products and increasing people's confidence in the safety and efficacy of products.
However, the high cost of development and the complex regulatory environment can hinder the growth of the market during the forecast period.
Neurotechnology Market Trends
Neurostimulation Devices Segment is Expected to Witness Significant Growth during the Forecast Period
Neurostimulation devices deliver electrical or magnetic stimulation to specific nerves or areas of the nervous system in order to modulate neural activity, which can be used for various therapeutic purposes, including pain management, treatment of neurological disorders, neuromodulation, and rehabilitation. These devices can be implanted or be used as external gadgets. They are designed to provide targeted and personalized therapy to patients suffering from chronic pain, movement disorders, psychiatric conditions, and other neurological conditions.
The major factors that are driving the growth of this market segment include rapidly rising technological advancements, patients' preferences for non-invasive and minimally invasive treatments, and the rising number of product launches. Neurostimulation devices are being increasingly used for a broader range of indications beyond traditional neurological disorders. They are now being explored for applications in pain management, obesity treatment, addiction therapy, and psychiatric disorders such as depression and anxiety, thereby expanding the addressable market and contributing to its rapid growth.
According to an article published by the American Academy of Neurology Journal in January 2022, neurostimulation devices are being approved for the treatment of movement disorders, epilepsy, pain, and depression. As per the same source, by 2035, advancements in the understanding of neuroanatomical networks, the mechanism of action in stimulation, the expanding scope of material science, miniaturization, energy storage facilities, and better delivery will lead to a higher usage of neurostimulation devices, which will significantly drive the growth of this market segment during the forecast period.
In addition, an increase in strategic activities by the key players, such as product launches and collaborations, and a surge in product approvals by the regulatory authorities will bolster the growth of the market. In January 2022, TensCare showcased its latest in transcutaneous electrical nerve stimulation at Arab Health 2022. This device is widely used for drug-free pain relief for the long-term treatment of chronic pain conditions such as diabetic neuropathy, backache, sciatica, osteoarthritis, and post-childbirth trauma.
In November 2022, ElectroCore Inc., a commercial-stage bioelectronic medicine company, received a unique national product code number from the Belgian Pharmaceutical Association for its gamma-core sapphire non-invasive vagus nerve stimulator (nVNS). Furthermore, in August 2022, Medtronic Private Limited launched the SenSight directional lead system for deep brain stimulation (DBS) therapy to treat symptoms associated with movement disorders and epilepsy in India.
Factors such as the rising number of product launches and rapid technological advancements are expected to drive the growth of the market during the forecast period.
North America is Expected to Witness Significant Growth during the Forecast Period
The North American market is likely to witness healthy growth, owing to factors like the rising geriatric population, increasing neurological disorders, and strategic product launches by the key players.
A significant number of people in the region are suffering from various neurological disorders, which is estimated to create a high demand for neurotechnology devices during the forecast period. According to the data updated by the Parkinson's Foundation in January 2024, approximately 90,000 people in the United States are diagnosed with Parkinson's disease every year, and around 1.2 million people across the country are expected to live with Parkinson's disease by the end of 2030.
According to the data published by Statistics Canada in January 2024, approximately 750,000 Canadians suffer from Alzheimer's disease or other forms of dementia. In an article published by The Journal of Prevention of Alzheimer's Disease in October 2022, Alzheimer's disease and other dementia-related diseases were ranked second among all neurological disorders that result in disability-adjusted life years (DALY) in Mexico. The rising number of neurological disorders in North America is projected to fuel the development of innovative neurotechnologies aimed at diagnosing and treating neurological disorders. These developments are expected to drive the growth of the North American market.
Furthermore, the rising number of product developments is projected to accelerate the growth of the market during the forecast period. In January 2024, Abbott received approval from the FDA to launch the Liberta RC DBS system, one of its smallest rechargeable deep brain stimulation (DBS) devices with remote programming, to treat people living with movement disorders.
In January 2022, Nevro Corp. received the FDA's approval for expanded labeling of its Senza Spinal Cord Stimulation (SCS) system for treating non-surgical refractory back pain (NSRBP). In January 2022, Medtronic received the FDA's approval for its Intellis rechargeable neurostimulator and Vanta recharge-free neurostimulator for treating chronic pain associated with diabetic peripheral neuropathy (DPN).
Thus, factors such as the rising instances of neurological disorders and the increasing number of product developments are projected to drive the growth of the regional market during the forecast period.
Neurotechnology Industry Overview
The neurotechnology market is semi-consolidated in nature due to the presence of several companies operating globally and regionally. The major players are continuously involved in various strategic activities, such as mergers, acquisitions, and partnerships. The competitive landscape includes a strong presence of local and regional companies that hold market shares and are well known, including Abbott, Boston Scientific, Medtronic, and GE Healthcare.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Prevalence of Neurological Disorders
- 4.2.2 Surging Advancements in Neuroscience and Technology
- 4.2.3 Growing Demand for Improved Treatment Options
- 4.3 Market Restraints
- 4.3.1 High Cost of Neurotechnology Devices
- 4.3.2 Stringent Regulatory Framework
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Product Type
- 5.1.1 Neuroprosthetics
- 5.1.2 Neurostimulation Devices
- 5.1.3 Brain-Computer Interfaces
- 5.1.4 Other Products
- 5.2 By Application
- 5.2.1 Neurodegenerative Disorders
- 5.2.2 Neuropsychiatric Disorders
- 5.2.3 Chronic Pain Management
- 5.2.4 Other Applications
- 5.3 By End User
- 5.3.1 Hospitals and Clinics
- 5.3.2 Research Institutes and Academic Centers
- 5.3.3 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Medtronic
- 6.1.2 Abbott Laboratories
- 6.1.3 Boston Scientific
- 6.1.4 Siemens Healthineers
- 6.1.5 GE Healthcare
- 6.1.6 LivaNova PLC
- 6.1.7 NeuroPace
- 6.1.8 Neuronetics
- 6.1.9 Koninklijke Philips N.V.
- 6.1.10 Elekta AB

















