
|
시장보고서
상품코드
1521788
트랜스티레틴 아밀로이드증 치료 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2024-2029년)Transthyretin Amyloidosis Treatment - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
트랜스티레틴 아밀로이드증 치료 시장 규모는 2024년 53억 달러로 추정되며, 2029년에는 109억 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 15.40%의 CAGR로 성장할 것으로 예상됩니다.
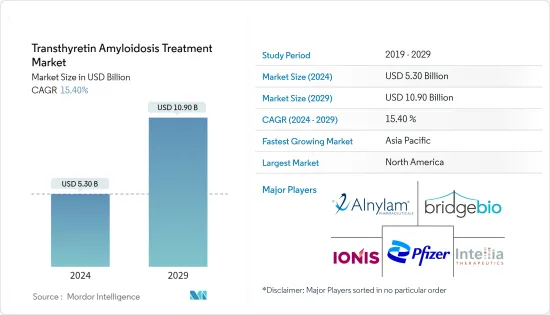
트랜스티레틴 아밀로이드증 치료 시장은 노인 인구 증가, 아밀로이드 경쇄(AL) 위험인자 증가, 연구개발(R&D) 증가, 일반인의 질환에 대한 인식 증가 등으로 인해 큰 성장이 예상됩니다. 노화는 체내 단백질 구조와 기능의 변화와 관련이 있습니다. 주로 간에서 생산되는 수송 단백질인 트랜스시레틴은 시간이 지남에 따라 오접힘이 발생합니다. 이 오접힘은 비정상적인 단백질 응집체가 형성되어 조직에 축적되어 장기 기능 장애를 유발하는 아밀로이드증의 발병에 중요한 요인입니다.
또한, 형질세포의 이상을 특징으로 하는 형질세포 이상증은 아밀로이드증의 아형인 아밀로이드 경쇄(AL) 아밀로이드증의 위험인자로 알려져 있습니다. 다발성 골수종과 같은 질환을 포함한 형질세포이형성증의 발병 위험은 나이가 들수록 형질세포의 기능 및 증식 이상이 발생하기 쉬워지는 경향이 있습니다. 예를 들어, WHO가 2023년 10월에 발표한 자료에 따르면, 2024년까지 WHO 유럽 지역에서는 65세 이상 인구가 15세 미만 인구보다 많을 것으로 추정됩니다. 이는 젊은 층에 비해 노인 인구가 많다는 것을 의미하며, 이는 아밀로이드 경쇄(AL) 아밀로이드증의 위험을 증가시킬 수 있기 때문에 조사 기간 동안 시장 성장을 촉진할 것으로 보입니다.
또한, 연구개발의 증가와 질병에 대한 인식의 증가는 트랜스실레틴 아밀로이드증 치료제 시장의 성장을 촉진하는 중요한 요인입니다. 제약 및 생명공학 기업들은 트랜스티레틴 아밀로이드증에 대한 혁신적인 표적 치료제를 개발하기 위해 연구개발에 많은 자원을 투입하고 있습니다. 예를 들어, 2024년 2월 아스트라제네카는 트랜스실레틴 매개 아밀로이드성 심근증(ATTR-CM) 성인 환자를 대상으로 한 아코라미디스의 일본 임상 3상 시험에서 좋은 결과를 보고했습니다. 마찬가지로 2023년 3월, 이오니스 파마슈티컬스(Ionis Pharmaceuticals)는 미국 식품의약국(FDA)이 유전성 트랜스시레틴 매개 아밀로이드성 다발성 신경병증(ATTRv-PN) 치료용 항센스 약물인 에프론타센(Eplantasen)의 신약승인신청(NDA)을 승인했다고 보고했습니다. 보고하였습니다. 이러한 사례들은 트랜스티레틴 아밀로이드증 치료와 관련된 연구개발의 성장을 보여주며, 예측 기간 동안 시장이 크게 성장할 것으로 예상됩니다.
따라서 노인 인구의 증가, AL의 위험 요인, 연구 개발 활동의 증가는 트랜스시레틴 아밀로이드증 치료 시장의 성장을 촉진하는 몇 가지 주요 요인입니다. 그러나 이 치료와 관련된 높은 비용은 예측 기간 동안 시장을 억제할 것으로 예상됩니다.
트랜스티레틴 아밀로이드증 치료 시장 동향
예측 기간 동안 유전성 트랜스티레틴 아밀로이드증 부문이 큰 비중을 차지할 것으로 예상
유전성 트랜스실레틴 아밀로이드증(hATTR)은 트랜스실레틴(TTR) 유전자의 돌연변이로 인해 말초(신체 및 자율신경계) 장애를 동반하는 희귀하고 심각한 다계통성 질환입니다. 유전성 트랜스시레틴 아밀로이드증(hATTR)은 유전적 특성으로 인해 표적 치료 노력에 일관성 있는 환자 기반을 제공하기 때문에 큰 시장 점유율을 차지할 것으로 예상됩니다. 이 부문의 성장을 촉진하는 요인으로는 인식 제고 프로그램을 통한 hATTR에 대한 인식 제고, 연구 투자 증가, 제품 가용성을 높이기 위한 파트너십, 제품 승인 및 출시와 같은 시장 기업의 전략 등이 있습니다.
질병에 대한 인식이 높아지면서 효과적인 치료에 대한 수요가 증가함에 따라 이 부문의 성장에 큰 도움이 될 것입니다. 의료 전문가와 일반인들이 증상, 위험 요인, 진단의 발전에 대한 지식이 증가함에 따라 적극적인 관리에 대한 중요성이 커지고 있습니다. 예를 들어, 2024년 3월 알나이람은 가족들이 유전성 ATTR(hATTR) 아밀로이드증 발병 위험을 이해하기 위해 가족 구성원들이 주치의와 건강 이력에 대해 대화를 나누도록 장려하는 '가족 건강 이력 로드트립'을 시작했습니다. 이러한 인식 개선은 다양한 제품을 통한 조기 진단 및 치료에 도움이 되기 때문에 조사 기간 동안 이 분야의 성장을 촉진하고 있습니다.
또한, 제휴 및 제품 승인과 같은 시장 기업의 전략은 시장에서 제품의 가용성을 높여 해당 부문의 성장을 촉진할 수 있습니다. 예를 들어, 2024년 1월 아스트라제네카와 이오니스는 Orsini Specialty Pharmacy를 유전성 트랜스시레틴 매개 아밀로이드성 다발성 신경병증 치료제로 FDA 승인을 받은 WAINUA(eprontazene)의 독점 전문약국 파트너로 선정했습니다. 선정하였습니다. 또한, 2022년 8월 알나이람 파마슈티컬스는 유전성 트랜스시레틴 아밀로이드증으로 인한 신경통에 사용할 수 있는 온파티로(Onpatro)가 FDA의 승인을 받았다고 발표했습니다. 이러한 전략적 활동은 제품의 가용성을 높이고 예측 기간 동안 이 분야의 성장을 촉진할 것으로 예상됩니다.
따라서, 유전성 트랜스티레틴 아밀로이드증 분야는 시장 기업들의 인식 개선과 전략적 활동으로 인해 예측 기간 동안 큰 시장 점유율을 차지할 것으로 예상됩니다.
예측 기간 동안 북미가 가장 큰 시장 점유율을 기록할 것으로 예상
북미는 선진화된 의료 인프라와 트랜스실레틴 아밀로이드증 질환의 발병률 증가 등의 요인으로 인해 가장 큰 시장 점유율을 차지할 것으로 예상됩니다. 탄탄한 연구 역량, R&D 투자 확대, 임상시험 인프라가 이 지역의 시장 성장을 견인하고 있습니다.
트랜스티레틴 아밀로이드증(ATTR)에 대한 첨단 치료 제품 개발에 대한 새로운 투자가 시장 성장을 촉진할 것으로 예상됩니다. 예를 들어, 2023년 5월 미국 국립보건원(NIH)은 ATTR 아밀로이드증 치료제 개발을 위해 아밀로이드증 연구 컨소시엄에 4만 달러를 지원했습니다. 이러한 요인들은 예측 기간 동안 시장 성장을 촉진할 것으로 예상됩니다.
또한, 제품 승인 및 규제 당국의 적극적인 권고와 같은 시장 기업의 전략이 성장함에 따라 이 지역에서 치료 제품의 사용량이 증가할 것입니다. 예를 들어, 2024년 4월 Alnylam Canada ULC는 CADTH(Canadian Agency for Drugs and Technologies in Health)로부터 AMVUTTRA(vutrisiran injection)의 상환에 대한 긍정적인 권고를 받았습니다. 받았습니다. 마찬가지로 2023년 10월, AMVUTTRA는 유전성 트랜스시레틴 매개 아밀로이드증(hATTR 아밀로이드증) 성인 환자의 1기 또는 2기 다발성 신경병증 치료제로 캐나다에서 판매 승인을 받았습니다. 따라서 적극적인 추천과 승인은 이 지역에서 제품의 사용과 가용성을 증가시켜 예측 기간 동안 이 지역의 시장 성장을 촉진할 것입니다.
트랜스티레틴 아밀로이드증 치료 산업 개요
트랜스티레틴 아밀로이드증 치료 시장은 세계 및 지역적으로 사업을 전개하는 제한된 수의 기업이 존재하기 때문에 시장 내 통합성이 높은 편입니다. 경쟁 상황에는 Pfizer Inc., Ionis Pharmaceuticals, Alnylam Pharmaceuticals Inc, Intellia Therapeutics Inc, BridgeBio Inc. 등 시장 점유율과 인지도가 높은 국제 기업 및 현지 기업 분석이 포함됩니다.
기타 혜택:
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 소개
- 조사 가정과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 노년 인구 증가와 아밀로이드 경쇄(AL) 위험인자
- 연구개발(R&D) 증가와 질환에 대한 인식 확산
- 시장 성장 억제요인
- 트랜스티레틴 아밀로이드증 치료에 따른 고비용
- Porter's Five Forces 분석
- 신규 참여업체의 위협
- 구매자/소비자의 협상력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업 간의 경쟁 강도
제5장 시장 세분화(시장 규모 - 금액)
- 유형별
- ATTR-CM(트랜스티레틴 아밀로이드 심근병증)
- ATTR-PN(트랜스티레틴 아밀로이드 다발성 신경병증)
- 치료법별
- 표적요법
- 지지요법
- 질환 유형별
- 유전성 아밀로이드증
- 야생형 아밀로이드증
- 기타 질환 유형
- 판매 채널별
- 병원 약국
- 소매 약국
- 기타 유통 채널
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 세계 기타 지역
- 북미
제6장 경쟁 상황
- 기업 개요
- Pfizer Inc
- Ionis Pharmaceuticals
- Alnylam Pharmaceuticals Inc.
- BridgeBio Inc
- Intellia Therapeutics Inc.
- Prothena
- Alexion Pharmaceuticals
- Millennium Pharmaceuticals
- Corino Therapeutics Inc.
- Takeda Pharmaceutical Company Limited
- Oncopeptides
- SOM BIOTECH
제7장 시장 기회와 향후 동향
ksm 24.08.01The Transthyretin Amyloidosis Treatment Market size is estimated at USD 5.30 billion in 2024, and is expected to reach USD 10.90 billion by 2029, growing at a CAGR of 15.40% during the forecast period (2024-2029).
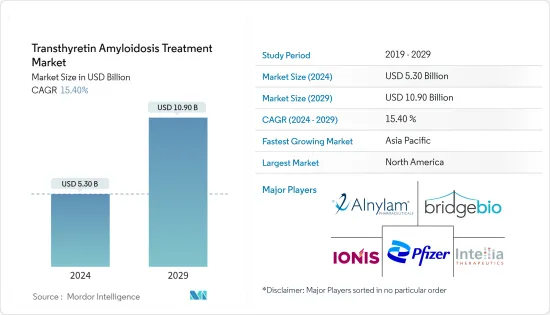
The transthyretin amyloidosis treatment market is expected to grow significantly due to the growing geriatric population and risk factors for the amyloid light chain (AL), increasing research and developments (R&D), and raising public disease awareness. Aging is associated with changes in the body's protein structure and function. Transthyretin, a transport protein primarily produced in the liver, undergoes misfolding over time. This misfolding is a critical factor in the development of amyloidosis, where abnormal protein aggregates form and accumulate in tissues, leading to organ dysfunction.
In addition, plasma cell dyscrasia, a condition characterized by abnormal plasma cells, is a known risk factor for amyloid light chain (AL) amyloidosis, a subtype of amyloidosis. The risk of developing plasma cell dyscrasias, including conditions like multiple myeloma, tends to increase as older individuals are more susceptible to abnormalities in plasma cell function and proliferation. For instance, according to the data published by the WHO in October 2023, the population of individuals aged over 65 years is estimated to outnumber those under the age of 15 years in the WHO European region by 2024. This shows a significant number of people in the aging population compared to those of a young age, which may increase the risk of amyloid light chain (AL) amyloidosis, thereby boosting market growth over the study period.
Furthermore, increasing R&D and growing disease awareness are other significant factors driving the growth of the transthyretin amyloidosis treatment market. Pharmaceutical and biotechnology firms are committing substantial resources to R&D to pioneer innovative and targeted treatments for transthyretin amyloidosis. For instance, in February 2024, AstraZeneca reported positive high-level results from the Japan Phase III trial of acoramidis in adults with transthyretin-mediated amyloid cardiomyopathy (ATTR-CM). Similarly, in March 2023, Ionis Pharmaceuticals reported the acceptance of a New Drug Application (NDA) for eplontersen, an antisense medicine for the treatment of hereditary transthyretin-mediated amyloid polyneuropathy (ATTRv-PN) by the Food and Drug Administration. These instances show the growing R&D on treating transthyretin amyloidosis, which is expected to have significant growth in the market over the forecast period.
Therefore, the growing geriatric population, risk factors for AL, and increasing R&D activities are some major factors driving the growth of the transthyretin amyloidosis treatment market. However, the high cost associated with this treatment is expected to restrain the market over the forecast period.
Transthyretin Amyloidosis Treatment Market Trends
The Hereditary Transthyretin Amyloidosis Segment is Expected to Hold a Significant Share Over the Forecast Period
Hereditary transthyretin amyloidosis (hATTR) is a rare and severe, heterogeneous multisystem condition with prevalent peripheral (both somatic and autonomic) nervous system impairment due to mutations in the transthyretin (TTR) gene. Hereditary transthyretin amyloidosis (hATTR) is expected to hold a significant market share due to its hereditary characteristics, providing a consistent and identifiable patient base for targeted therapeutic endeavors. The factors driving the growth of this segment include growing awareness of hATTR through awareness programs, growing research investments, and market player strategies like partnerships, product approvals, and launches, which increase the availability of the products.
The increasing disease awareness significantly drives the segment growth as it increases the demand for effective treatments. As healthcare professionals and the general public become more informed about the symptoms, risk factors, and diagnostic advancement related to conditions, there is a growing emphasis on proactive management. For instance, in March 2024, Alnylam Pharmaceuticals Inc. launched the Family Health History Road Trip to encourage conversations between family members about their health history with their doctor to understand their risk for developing hereditary ATTR (hATTR) amyloidosis. This awareness helps in early diagnosis and treatment of the disease using various products, thereby boosting segment growth over the study period.
Furthermore, the strategies of market players, like partnerships and product approvals, increase the availability of the products in the market, thereby boosting segment growth. For instance, in January 2024, AstraZeneca and Ionis selected Orsini Specialty Pharmacy as the exclusive specialty pharmacy partner for WAINUA (eplontersen), an FDA-approved treatment for adults living with hereditary transthyretin-mediated amyloid polyneuropathy. In addition, in August 2022, Alnylam Pharmaceuticals Inc. stated that the FDA approved Onpattro, which can be used for nerve pain caused by hereditary transthyretin amyloidosis. These strategic activities are expected to increase the availability of the products and boost the segment growth over the forecast period.
Therefore, due to the growing awareness and strategic activities of market players, the hereditary transthyretin amyloidosis segment is expected to hold a significant market share over the forecast period.
North America is Expected to Record the Largest Share in the Market Over the Forecast Period
North America is expected to have the largest share in the market due to factors such as advanced healthcare infrastructure and the increasing incidence of transthyretin amyloidosis disease in the region. Established research capabilities, growing investments for research and development, and clinical trial infrastructure are driving the region's market growth.
New investments in developing advanced treatment products for transthyretin amyloidosis (ATTR) are expected to boost the market growth. For instance, in May 2023, the National Institutes of Health (NIH) awarded the Amyloidosis Research Consortium with USD 40,000 toward advancing ATTR amyloidosis drug development. These factors are expected to propel the market growth over the forecast period.
Additionally, growing market player strategies, such as product approvals and positive recommendations from the regulatory authorities, increase the usage of treatment products in the region. For instance, in April 2024, Alnylam Canada ULC received a positive recommendation for reimbursement from the Canadian Agency for Drugs and Technologies in Health (CADTH) for AMVUTTRA (vutrisiran injection). Similarly, in October 2023, AMVUTTRA was authorized for sale in Canada to treat stage 1 or stage 2 polyneuropathy in adult patients with hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis). Hence, the positive recommendations and approvals increase the usage and availability of products in the region, thereby boosting the region's market growth over the forecast period.
Transthyretin Amyloidosis Treatment Industry Overview
The transthyretin amyloidosis treatment market is moderately consolidated in nature due to the presence of limited companies operating globally as well as regionally. The competitive landscape includes an analysis of a few international as well as local companies that hold market shares and are well known, including Pfizer Inc., Ionis Pharmaceuticals, Alnylam Pharmaceuticals Inc., Intellia Therapeutics Inc., and BridgeBio Inc.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Geriatric Population and Risk Factors for Amyloid Light Chain (AL)
- 4.2.2 Increasing Research and Development (R&D) and Growing Disease Awareness
- 4.3 Market Restraints
- 4.3.1 High Cost Associated with the Transthyretin Amyloidosis Treatment
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Type
- 5.1.1 ATTR-CM (Transthyretin Amyloid Cardiomyopathy)
- 5.1.2 ATTR-PN (Transthyretin Amyloid Polyneuropathy)
- 5.2 By Therapy
- 5.2.1 Targeted Therapy
- 5.2.2 Supportive Therapy
- 5.3 By Disease Type
- 5.3.1 Hereditary Amyloidosis
- 5.3.2 Wild-type Amyloidosis
- 5.3.3 Other Disease Type
- 5.4 By Distribution Channel
- 5.4.1 Hospitals Pharmacy
- 5.4.2 Retail Pharmacies
- 5.4.3 Other Distribution Channel
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Rest of the World
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Pfizer Inc
- 6.1.2 Ionis Pharmaceuticals
- 6.1.3 Alnylam Pharmaceuticals Inc.
- 6.1.4 BridgeBio Inc
- 6.1.5 Intellia Therapeutics Inc.
- 6.1.6 Prothena
- 6.1.7 Alexion Pharmaceuticals
- 6.1.8 Millennium Pharmaceuticals
- 6.1.9 Corino Therapeutics Inc.
- 6.1.10 Takeda Pharmaceutical Company Limited
- 6.1.11 Oncopeptides
- 6.1.12 SOM BIOTECH















