
|
시장보고서
상품코드
1850985
암 치료 시장 : 점유율 분석, 산업 동향, 통계, 성장 예측(2025-2030년)Global Cancer Therapy - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
암 치료 시장은 2025년에 2,436억 2,000만 달러, 2030년에는 4,039억 9,000만 달러에 이르고, 2025-2030년의 CAGR은 10.64%를 나타낼 전망입니다.

유전체 프로파일링의 진전, 종양 진단 인가의 신속화, 세포 및 유전자 치료 파이프라인의 확대가, 암 치료 시장을 두자리 성장으로 끌어 올리고 있습니다. 주요 제약 회사는 바이오 마커에 중점을 둔 포트폴리오를 선호하고 있으며 아시아 건강 관리 투자는 혁신적인 요법의 지역 도입을 가속화하고 있습니다. 또한 규제 당국도 유연성을 늘리고 있으며 실제 임상 증거가 승인까지의 기간을 단축할 수 있게 되었습니다. 이러한 기회에도 불구하고, 암 치료 시장은 바이러스 벡터 공급망의 한계와 지속적인 금전적 독성에 직면하고 있으며, 이 두 가지가 당면의 보급률을 낮출 수 있습니다.
세계 암 치료 시장 동향과 통찰
종양 진단 요법 및 바이오마커 주도형 요법
2024년까지 fam-trastuzumab deruxtecan-nxki, repotrectinib 등 8가지 종양 진단제가 승인되었습니다. 실제 데이터는 21.5%의 환자가 잠재적인 후보임을 나타내며, 암 치료 시장은 확대되고 적응 시험 설계는 장려되고 있습니다. 제약회사는 현재 이러한 프레시젼 메디신의 길을 따르고, 또한 개발 후기의 위축 리스크를 경감하기 위해 동반진단약을 개발 초기 단계에서 우선적으로 사용하고 있습니다. 이 접근법은 또한 대응 가능한 환자 풀을 더욱 확장시킬 수 있는 종양 횡단적 병용 요법 연구를 촉진합니다.
파이프라인 성장을 가속화하는 세포 및 유전자 치료
세포 및 유전자 치료에 대한 투자는 2024년에는 30% 증가한 152억 달러로 급증했습니다. 2,000개 이상의 임상시험과 3,000개 이상의 개발자가 이 치료법의 기세를 뒷받침하고 있습니다. 2024년 2월에 승인된 lifileucel(Amtagvi)은 고형암에 대한 최초의 종양 침윤성 림프구 요법으로 31.5%의 객관적 효과를 달성했습니다. 현재 주요 제약기업 15개사 중 13개사가 CGT 전문부문을 가지고 있으며, 이 파괴적 플랫폼에 대한 장기적인 헌신을 반영하고 있습니다.
심각화하는 경제적 독성
암 환자의 75%가 자기 부담액의 보조를 요구하고 있으며, 42.0%가 심각한 경제적 부담을 호소하고 있습니다. 백혈병에서는 이식 자격이 있는 환자의 75.0%가 치료를 늦추거나 중단하는 고통을 경험합니다. 가계 규모가 큰 젊은 성인은 불균형에 영향을 받고 종종 복약 준수를 낮춥니다. 체계적인 경제적 고통을 스크리닝하는 의료 시스템은 거의 없으며, 고비용의 처방이 표준 치료가 되는 가운데, 접근을 보호하기 위한 정책적 개입의 여지가 남아 있습니다.
부문 분석
표적 요법은 특정 분자 드라이버를 저해하는 약제에 대한 임상의의 강한 신뢰를 반영하여 2024년 암 치료 시장의 37.0%를 차지했습니다. 2017년 이후 승인된 8종의 종양 진단제가 이러한 우위를 지탱하고 있으며, 항체 약물 복합체와 티로신 키나아제 억제제는 적응 확대를 계속하고 있습니다. 면역요법은 라이프로이셀이나 CAR-T의 개량 등 획기적인 진보에 힘입어 가장 급성장하고 있는 분야입니다. 이와는 대조적으로, 화학요법의 역할은 정밀한 요법에서 병용 요법으로 이동하여 분자 유도 치료로의 진화를 강화하고 있습니다.
면역요법의 암 치료 시장 규모는 2024년 580억 달러에서 2030년 1,200억 달러로 확대될 것으로 예측되며, CAGR은 14.9%를 나타낼 전망입니다. 체크포인트 억제제가 매출을 이끌고 있지만, 차세대 이중특이적 항체는 추가 성장을 가져옵니다. 경쟁의 격렬함은 높고, 500 이상의 PD-1/L1 시험이 진행중입니다. 각 회사는 신규 타겟(TIGIT, LAG-3 등)과 피하 투여 제제에 의한 브랜드 라이프 사이클의 연장으로 차별화를 도모하고 있습니다.
유방암 치료는 2024년에는 암 치료 시장 규모의 18.2%를 차지하며, 이 카테고리가 임상 혁신의 기수로서의 지위를 굳힙니다. 아시아는 세계 유방암 이환율의 절반 가까이를 차지하고 있으며 호르몬 수용체 양성 아형에 대한 지역 특이적인 임상시험을 뒷받침하고 있습니다. CAR-T나 이중 특이성 항체에 밀려, 혈액암이 이에 이어집니다. FDA가 2025년 3월에 승인한 오베카부타진 오토로이셀은 난치성 B세포성 ALL에서 63%의 완전한 관해율을 달성하여 세포 치료의 변화 가능성을 보여줍니다.
폐암에서는 ALK, EGFR, ROS1 검사 양성률이 제일 선택이 되어 경험적 화학요법에서 유전자형에 적합한 처방으로의 꾸준한 전이를 지지하고 있습니다. 종양 돌연변이 부하와 KRAS G12C 타겟팅은 Precision-on-Cology Toolkit의 폭을 더욱 넓혀 암 치료 시장의 이 높은 인시던스 부문에서 수요의 성장을 지원합니다.
지역 분석
북미는 2024년 암 치료시장의 43.0%를 차지했으며 깊은 임상시험 파이프라인과 폭넓은 보험 적용에 지지되고 있습니다. 미국은 퍼스트 인 클래스 승인으로 선도하고 있으며, FDA는 2024년 동안 29건의 암 치료제 신청을 승인했습니다. 그럼에도 불구하고 환자의 자기 부담액은 가구 소득의 20% 이상의 경우가 많으며, 가치 기반 가격 설정에 대한 사회적 논의가 높아지고 있습니다.
아시아는 가장 급성장하고 있는 지역으로, 암 치료 시장은 2030년까지의 CAGR이 11.2%가 될 것으로 예상되고 있습니다. 중국의 암 연구생산액은 현재 미국을 상회하고 있습니다. 우선심사 바우처나 집중조달개혁 등 정부의 인센티브는 가격 인플레이션을 억제하면서 현지 기술 혁신을 가속시키는 것을 목적으로 하고 있습니다. 동남아시아에서는 2050년까지 연간 203만명의 신규 사례가 발생할 것으로 예측되고 있으며, 스크리닝 프로그램이나 분자 생물학적 검사에 대한 액세스 확대가 급선무가 되고 있습니다.
유럽은 국민 모두 보험 제도와 공동 연구 네트워크에 힘입어 암 치료 업계에서 큰 점유율을 유지하고 있습니다. 유럽 의약품청은 최근 CDx 평가에 관한 통일 지침을 발표하고 정밀의료의 전개를 촉진하고 있습니다. 한편, 중동 및 아프리카, 남미는 신흥 클러스터입니다. 이러한 시장은 기술 이전 파트너십에 투자하고 생물 제제의 현지 생산을 촉진함으로써 저렴한 가격과 공급의 탄력성을 높입니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건과 시장의 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 상황
- 시장 개요
- 시장 성장 촉진요인
- 종양 비의존형 및 바이오마커 주도형 치료법의 도입을 가속
- 세포 및 유전자 치료 파이프라인의 확대, 세계에서 2,000건을 넘는 임상시험 실시중
- 일본과 중국에서의 신속 승인 취득을 위한 실제 증거의 활용 확대
- 동반진단약의 공동 발매가 급증해, 표적약 시장 투입까지의 시간을 단축
- 미국의 민간 보험 시스템에서 종양학의 약제 번들 모델이 보급
- 아시아태평양에서의 계약 개발과 제조의 확대에 의해 비용 효율적인 생산 촉진
- 시장 성장 억제요인
- 치료 포기로 이어지는 경제적 독성 증가
- 바이러스 벡터의 제조 능력의 병목이 세포 요법 공급을 제한
- HTA가치평가기준의 차이가 시장 진입을 지연시킨다
- 고형 종양에서 장기 효능을 손상시키는 면역 요법 내성 메커니즘
- 규제 전망
- 기술의 전망
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계
제5장 시장 규모와 성장 예측
- 치료유형별
- 화학요법
- 표적요법
- 면역요법
- 호르몬 요법
- 기타 치료의 유형
- 암유형별
- 혈액암
- 유방암
- 전립선암
- 소화기암
- 부인과암
- 호흡기암/폐암
- 기타 암 유형
- 투여 경로별
- 정맥 내
- 구강
- 피하
- 종양 내
- 최종 사용자별
- 병원
- 전문 클리닉
- 암 및 방사선 치료 센터
- 재택 케어 설정
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 한국
- 호주
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 시장 집중도
- 전략적 동향
- 시장 점유율 분석
- 기업 프로파일
- F. Hoffmann-La Roche AG
- Bristol Myers Squibb Company
- Johnson & Johnson Services Inc.(Janssen)
- Merck & Co., Inc.
- AstraZeneca PLC
- Novartis AG
- Pfizer Inc.
- Amgen Inc.
- AbbVie Inc.
- GSK PLC
- Takeda Pharmaceutical Company Limited
- Astellas Pharma Inc.
- Gilead Sciences Inc.(Kite Pharma)
- Seagen Inc.
- Regeneron Pharmaceuticals, Inc.
- BeiGene Ltd.
- Exelixis, Inc.
- Eli Lilly and Company
- Celldex Therapeutics Inc.
- Alaunos Therapeutics Inc.
제7장 시장 기회와 장래의 전망
SHW 25.11.11The cancer therapy market is valued at USD 243.62 billion in 2025 and is forecast to expand to USD 403.99 billion by 2030, reflecting a 10.64% CAGR for 2025-2030.
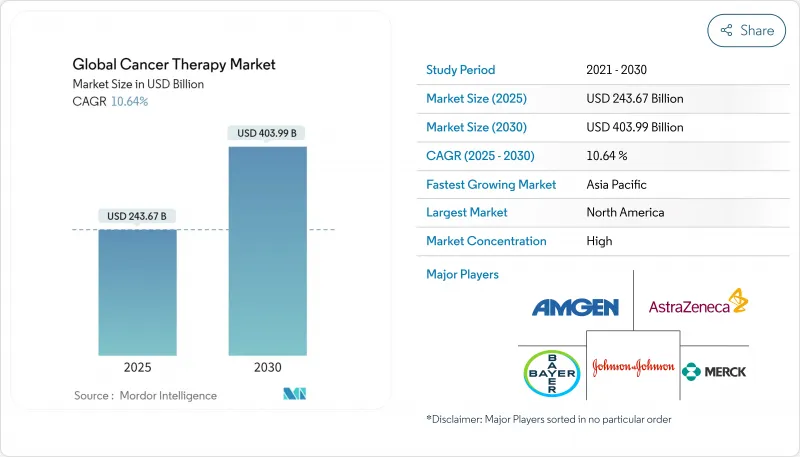
Advancing genomic profiling, faster tumor-agnostic approvals, and the expanding cell- and gene-therapy pipeline are propelling the cancer therapy market toward double-digit growth. Major pharmaceutical companies are prioritizing biomarker-driven portfolios, while Asia's healthcare investments accelerate regional uptake of innovative regimens. Regulatory agencies are also showing greater flexibility, enabling real-world evidence to shorten approval timelines. Despite these opportunities, the cancer therapy market faces supply-chain limits for viral vectors and persistent financial toxicity, both of which may temper near-term adoption rates.
Global Cancer Therapy Market Trends and Insights
Tumor-agnostic and biomarker-driven therapies
Approvals targeting genomic alterations rather than tissue of origin are reshaping clinical practice, with eight tumor-agnostic agents cleared by 2024, including fam-trastuzumab deruxtecan-nxki and repotrectinib. Real-world data show 21.5% of patients as potential candidates, widening the cancer therapy market and encouraging adaptive trial designs. Pharmaceutical firms now prioritize companion diagnostics at early development stages to align with these precision-medicine pathways and to reduce late-stage attrition risk. The approach is also catalyzing cross-tumor combination studies, which may further enlarge the addressable patient pool.
Cell & gene therapies accelerating pipeline growth
Investments in cell- and gene-based modalities jumped 30% in 2024 to USD 15.2 billion. More than 2,000 active trials and 3,000 developers underscore the modality's momentum. The February 2024 approval of lifileucel (Amtagvi) marked the first tumor-infiltrating lymphocyte therapy for solid tumors, achieving a 31.5% objective response. Thirteen of the 15 largest pharmaceutical firms now report dedicated CGT divisions, reflecting long-term commitment to this disruptive platform.
Escalating financial toxicity
Seventy-five percent of cancer patients seek copayment assistance, and 42.0% report severe financial strain. In leukemia, 75.0% of transplantation-eligible patients experience distress that can delay or curtail treatment. Younger adults with larger households are disproportionately affected, often reducing medication adherence. Few health systems offer systematic financial-distress screening, leaving room for policy interventions to safeguard access as high-cost regimens become standard of care.
Other drivers and restraints analyzed in the detailed report include:
- Real-world evidence fast-tracking regional access
- Companion diagnostics improve precision and speed
- Manufacturing capacity bottlenecks
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Targeted therapies commanded 37.0% of the cancer therapy market in 2024, reflecting strong clinician confidence in agents that inhibit specific molecular drivers. Eight tumor-agnostic approvals since 2017 anchor this dominance, while antibody-drug conjugates and tyrosine kinase inhibitors continue to expand indications. Immunotherapy is the fastest-growing segment, underpinned by breakthroughs such as lifileucel and CAR-T refinements. In contrast, chemotherapy's role is shifting toward combination backbone in precision regimens, reinforcing the evolution toward molecularly guided care.
The cancer therapy market size for immunotherapies is forecast to rise from USD 58 billion in 2024 to USD 120 billion by 2030, translating to a 14.9% CAGR. Checkpoint inhibitors lead unit sales, yet next-generation bispecific antibodies are adding incremental growth. Competitive intensity is high, with over 500 PD-1/L1 trials ongoing. Companies differentiate through novel targets (e.g., TIGIT, LAG-3) and subcutaneous formulations to extend brand life cycles.
Breast cancer treatments generated 18.2% of the cancer therapy market size in 2024, cementing the category's position as a bellwether for clinical innovation. Asia accounts for nearly half of global breast-cancer incidence, fueling region-specific trials on hormone-receptor-positive subtypes. Blood cancers follow, propelled by CAR-T and bispecific antibodies. The FDA's March 2025 approval of obecabtagene autoleucel, which delivered a 63% complete-remission rate in refractory B-cell ALL, illustrates the transformative potential of cell therapies.
In lung cancer, ALK, EGFR, and ROS1 test positivity rates now dictate first-line choice, anchoring a steady shift from empirical chemotherapy to genotype-matched regimens. Tumor mutational burden and KRAS G12C targeting further broaden the precision oncology toolkit, sustaining demand growth in this high-incidence segment of the cancer therapy market.
The Cancer Therapy Market Report is Segmented by Therapy Type (Chemotherapy, Targeted Therapy, Immunotherapy, and More), Cancer Type (Blood Cancer, Breast Cancer, Prostate Cancer, and More), Route of Administration (Intravenous, Oral, and More), End User (Hospitals, Specialty Clinics, and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America accounted for 43.0% of the cancer therapy market in 2024, supported by deep clinical-trial pipelines and broad insurance coverage. The United States has led in first-in-class approvals, with FDA clearing 29 oncology applications during 2024 alone. Even so, patient out-of-pocket costs often exceed 20% of household income, intensifying public debate on value-based pricing.
Asia is the fastest-growing region, with the cancer therapy market expected to post an 11.2% CAGR to 2030. China's oncology research output now surpasses that of the United States. Government incentives, such as priority review vouchers and centralized procurement reforms, aim to accelerate local innovation while containing price inflation. Southeast Asia anticipates 2.03 million new cases annually by 2050, underscoring pressing needs for screening programs and broader molecular testing access.
Europe retains a sizeable share of the cancer therapy industry, aided by universal health systems and collaborative research networks. The European Medicines Agency recently issued harmonized guidance on CDx assessment, facilitating precision-medicine rollouts. Meanwhile, the Middle East, Africa, and South America comprise emerging clusters. These markets invest in technology-transfer partnerships to boost local manufacturing of biologics, thereby enhancing affordability and supply resilience.
- Roche
- Bristol-Myers Squibb
- Johnson & Johnson Services Inc. (Janssen)
- Merck
- AstraZeneca
- Novartis
- Pfizer
- Amgen
- Abbvie
- GlaxoSmithKline
- Takeda Pharmaceuticals
- Astellas Pharma
- Gilead Sciences
- Seagen
- Regeneron Pharmaceuticals
- BeiGene Ltd.
- Exelixis, Inc.
- Eli Lilly and Company
- Celldex Therapeutic
- Alaunos Therapeutics Inc.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Accelerating Adoption of Tumor-Agnostic & Biomarker-Driven Therapies
- 4.2.2 Expansion of Cell & Gene Therapies Pipeline Crossing 2,000 Active Clinical Trials Globally
- 4.2.3 Rising Utilization of Real-World Evidence to Secure Accelerated Approvals in Japan and China
- 4.2.4 Surge in Companion Diagnostic Co-Launches Reducing Time-to-Market for Targeted Drugs
- 4.2.5 Oncology Drug Bundling Models Gaining Traction in US Commercial Payer Systems
- 4.2.6 Contract Development & Manufacturing Expansion in APAC Driving Cost-Effective Production
- 4.3 Market Restraints
- 4.3.1 Escalating Financial Toxicity Leading to Treatment Abandonment
- 4.3.2 Manufacturing Capacity Bottlenecks for Viral Vectors Limiting Cell Therapy Supply
- 4.3.3 Divergent HTA Value Assessment Criteria Delaying Market Access
- 4.3.4 Immunotherapy Resistance Mechanisms Undermining Long-Term Efficacy in Solid Tumors
- 4.4 Regulatory Outlook
- 4.5 Technological Outlook
- 4.6 Porter's Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitute Products
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Therapy Type
- 5.1.1 Chemotherapy
- 5.1.2 Targeted Therapy
- 5.1.3 Immunotherapy
- 5.1.4 Hormonal Therapy
- 5.1.5 Other Treatment Types
- 5.2 By Cancer Type
- 5.2.1 Blood Cancer
- 5.2.2 Breast Cancer
- 5.2.3 Prostate Cancer
- 5.2.4 Gastrointestinal Cancer
- 5.2.5 Gynecologic Cancer
- 5.2.6 Respiratory/Lung Cancer
- 5.2.7 Other Cancer Types
- 5.3 By Route of Administration
- 5.3.1 Intravenous
- 5.3.2 Oral
- 5.3.3 Subcutaneous
- 5.3.4 Intratumoral
- 5.4 By End User
- 5.4.1 Hospitals
- 5.4.2 Specialty Clinics
- 5.4.3 Cancer and Radiation Therapy Centers
- 5.4.4 Homecare Settings
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle-East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.4.1 F. Hoffmann-La Roche AG
- 6.4.2 Bristol Myers Squibb Company
- 6.4.3 Johnson & Johnson Services Inc. (Janssen)
- 6.4.4 Merck & Co., Inc.
- 6.4.5 AstraZeneca PLC
- 6.4.6 Novartis AG
- 6.4.7 Pfizer Inc.
- 6.4.8 Amgen Inc.
- 6.4.9 AbbVie Inc.
- 6.4.10 GSK PLC
- 6.4.11 Takeda Pharmaceutical Company Limited
- 6.4.12 Astellas Pharma Inc.
- 6.4.13 Gilead Sciences Inc. (Kite Pharma)
- 6.4.14 Seagen Inc.
- 6.4.15 Regeneron Pharmaceuticals, Inc.
- 6.4.16 BeiGene Ltd.
- 6.4.17 Exelixis, Inc.
- 6.4.18 Eli Lilly and Company
- 6.4.19 Celldex Therapeutics Inc.
- 6.4.20 Alaunos Therapeutics Inc.
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment

















