
|
시장보고서
상품코드
1537721
전임상 CRO : 시장 점유율 분석, 업계 동향 및 통계, 성장 전망(2024-2029년)Preclinical CRO - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
전임상 CRO 시장 규모는 2024년 71억 달러로 추정 및 예측되며, 2029년에는 101억 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 7.44%의 CAGR로 성장할 것으로 예상됩니다.
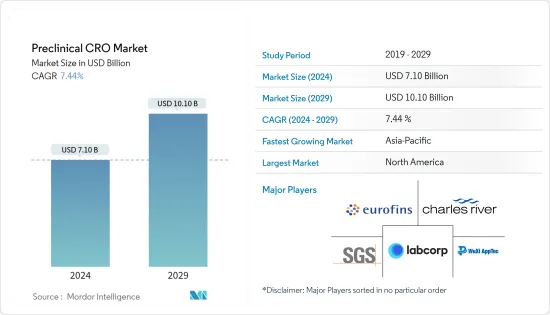
조사된 시장 성장의 주요 요인은 세계 연구개발(R&D) 지출 증가, 전임상시험 중인 의약품 수 증가, 2024년부터 2029년까지 만성질환 환자들의 의약품 복용 수요 증가로 나타났습니다.
생명과학 분야의 R&D 비용 증가와 이 분야에 대한 공공 및 민간 자금 투입이 시장 성장을 촉진하고 있습니다. 예를 들어, 인도 과학기술부는 2023-2024년 연방 예산에서 인도 생명공학국(DBT)에 400억 인도 루피(4억 2,720만 달러)의 예산을 배정했습니다. 자금이 크게 증가한 것은 주로 국내 R&D 비용의 증가에 기인합니다. 따라서 R & D 비용의 증가는 의약품 개발 프로세스의 전체 비용을 줄이기 위해 전임상 CRO 서비스의 채택을 증가시킬 것으로 예상됩니다.
또한 유럽제약협회(EFPIA)의 2022년 의약품 연구개발비는 440억 달러로 2021년 대비 4.6% 증가할 것으로 예상했습니다. 따라서 유럽의 R&D 비용 증가는 2024년부터 2029년까지 지출을 줄이기 위해 전임상 CRO 서비스에 대한 수요를 증가시킬 것으로 예상됩니다.
또한, 전임상 단계에 있는 의약품의 수가 증가함에 따라 개발 비용을 절감하고 임상시험 수행을 위한 의약품 승인 가능성을 높이기 위해 전임상 서비스 아웃소싱에 대한 수요가 증가할 것으로 예상됩니다. 예를 들어, ClinicalTrials.gov 2024의 업데이트된 데이터에 따르면, 2024년 1월 현재 전 세계적으로 약 47만 9,000건의 임상시험이 등록되어 있습니다. 이러한 임상시험의 대폭적인 증가는 전임상시험을 거쳐 신약 허가 신청(NDA)을 받은 치료제와 의료기기의 수를 반영합니다. 따라서 2024년부터 2029년까지 전임상시험 중인 의약품 수의 증가가 이 시장을 견인할 것으로 예상됩니다.
그러나 표준화 부족, 모니터링 문제, 엄격한 규제 정책이 시장 성장을 저해할 것으로 예상됩니다.
전임상 CRO 시장 동향
독성시험 부문, 2024-2029년 큰 폭의 성장 예상
독성시험은 화학제품이나 의약품 화합물이 인체에 미칠 수 있는 잠재적인 악영향을 평가하는 것입니다. 유해성 및 독성 유발 가능성을 포함하여 이러한 물질의 안전성 프로파일을 평가하기 위한 다양한 방법을 포함합니다. 제약 연구개발의 역동적인 환경 속에서 CRO(임상시험수탁기관)가 제공하는 전임상 독성시험 서비스에 대한 수요가 크게 증가하고 있습니다. 이러한 급증은 제약회사와 CRO의 협력 관계 확대, 독성 시험 과정의 효율성과 정확성을 높이는 기술 발전 등 다양한 요인에 기인합니다.
제약사와 CRO의 파트너십 확대로 독성 검사 서비스 수요가 증가할 것입니다. 제약사들이 의약품 개발 노력을 강화하면서 전임상 독성시험 수행에 필수적인 CRO의 전문성과 인프라를 필요로 하고 있습니다. 예를 들어, Immuter는 2023년 5월 Charles River Laboratories와 파트너십을 맺고 자가면역질환 치료제 후보물질 IMP761의 GLP(Good Laboratory Practice) 독성시험을 진행하기로 했습니다. 마찬가지로 2024년 3월 Badvus Research는 Southern Research와 전략적 제휴를 맺고 전임상 독성시험과 의약품 허가 절차에 집중하고 있습니다. 이러한 제휴는 CRO가 종합적인 독성 평가를 통해 의약품 개발 이니셔티브를 지원하고 규제 준수를 보장하는 데 있어 CRO가 매우 중요한 역할을 한다는 점을 강조하고 있습니다.
전임상 CRO의 독성시험은 제약회사가 후보물질의 안전성 프로파일을 평가하고 개발 파이프라인의 진행과 관련하여 정보에 입각한 의사결정을 내릴 수 있도록 하는 의약품 개발 프로세스의 중요한 요소로 작용하고 있습니다. 따라서 제약회사와 CRO의 수직적 협력 관계 강화는 시장 성장을 촉진할 것으로 보입니다.
북미가 2024년부터 2029년까지 시장에서 큰 비중을 차지할 것으로 예상
북미 전임상 CRO 시장은 자체 의약품 개발 및 탐색 비용 증가, 만성질환 유병률 증가, 임상시험의 복잡성 증가, 개발 파이프라인에 있는 수많은 임상시험 후보물질의 증가로 인해 수혜를 입을 것으로 예상됩니다.
심혈관질환, 당뇨병, 암, 신경질환 등 만성질환의 유병률 증가로 인해 새로운 치료법 및 치료제 개발이 요구되고 있습니다. 이러한 만성질환의 부담이 커지면서 제약회사들은 이러한 질병에 대한 유망한 후보물질을 조사하기 위해 전임상시험을 아웃소싱하고 있으며, 이는 조사기간 동안 시장 성장을 촉진할 것으로 예측됩니다. 예를 들어, 미국 암 협회에 따르면, 2022년 23만 6,740명의 폐암 환자가 보고된 반면, 2023년에는 23만 8,340명의 폐암 환자가 보고되는 등 폐암 유병률이 증가하고 있습니다. 이 데이터는 암 발병률이 급격히 증가하고 있으며, 2024년부터 2029년까지 암 발병률은 더욱 증가할 것으로 예상됩니다. 따라서 암 발병률의 증가는 첨단 치료제에 대한 수요를 촉진하고 전임상시험 활동을 촉진하여 연구 기간 동안 시장 성장을 지원할 것으로 예상됩니다.
또한, 자체적인 신약개발 비용이 높기 때문에 제약사들은 임상시험에 따른 비용 부담과 복잡성을 줄이기 위해 전임상시험 활동을 아웃소싱하는 경우가 많아지고 있습니다. 이러한 신약개발 비용의 증가는 필요한 인프라를 모두 갖춘 전임상시험수탁기관(CRO)의 임상시험 수행 수요를 촉진할 것으로 예상됩니다. 예를 들어, 세계보건기구(WHO)의 2022년 조사에 따르면, 신약 개발에 소요되는 평균 비용은 4,340만 달러에서 420만 달러에 달합니다. 이처럼 신약 개발에 소요되는 막대한 비용은 제약사를 대신해 임상시험 및 기타 연구 활동을 수행하는 전임상 CRO에 대한 수요를 촉진할 것으로 예상되는데, CRO는 연구 서비스를 수행할 수 있는 우수한 경험과 인프라를 보유하고 있기 때문입니다. 따라서 연구 활동을 수행하는 전임상 CRO에 대한 높은 수요는 연구 기간 동안 시장 성장을 뒷받침할 것으로 예상됩니다.
따라서 만성질환의 높은 유병률과 높은 자체 신약개발 및 시장개척 비용이 2024년부터 2029년까지 북미 연구 시장을 견인할 것으로 예상됩니다.
전임상 CRO 산업 개요
전임상 CRO 시장은 세계적으로 유명한 다양한 서비스 제공업체가 존재하기 때문에 경쟁이 치열하고 세분화되어 있습니다. 주요 시장 기업들은 솔루션과 서비스 범위를 확장하여 서로 경쟁하고 있습니다. 현재 몇몇 기업이 시장을 독점하고 있습니다. 주요 기업들은 세계 시장에서의 입지를 확보하기 위해 인수, 제휴, 고급 서비스 출시 등 다양한 전략적 제휴를 맺고 있습니다. 이들 기업에는 Eurofins Scientific, Charles River Laboratories, WuXi Apptec, Labcorp Drug Development, SGS SA 등이 있습니다.
기타 혜택
- 엑셀 형식의 시장 예측(ME) 시트
- 3개월간의 애널리스트 지원
목차
제1장 소개
- 조사 가정과 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 세계의 연구개발비 증가
- 전임상 시험중 의약품수 증가
- 만성질환 환자에 의한 의약품 수요 증가
- 시장 성장 억제요인
- 표준화와 모니터링의 결여
- 엄격한 규제 정책
- Porter's Five Forces 분석
- 신규 참여업체의 위협
- 구매자/소비자의 협상력
- 공급 기업의 교섭력
- 대체품의 위협
- 경쟁 기업 간의 경쟁 강도
제5장 시장 세분화(금액 기준 시장 규모 - 달러)
- 서비스별
- 독성 시험
- 바이오 분석 및 약물 대사·약물동태 시험
- 안전성 약리학
- 기타 서비스
- 모드 유형별
- 환자 유래 오가노이드(PDO) 모델
- 환자 유래 이종이식편(PDX) 모델
- 최종사용자별
- 바이오 제약회사
- 연구기관 및 대학
- 기타 최종사용자
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카공화국
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 상황
- 기업 개요
- Charles River Laboratories
- Labcorp Drug Development
- Thermo Fisher Scientific Inc.(Pharmaceutical Product Development(PPD))
- NorthEast BioAnalytical Laboratories LLC
- Parexel International Corporation
- Medpace
- Eurofins Scientific
- WuXi App Tec
- ICON PLC
- SGS SA
- PharmaLegacy Laboratories
- Altasciences Company Inc.
제7장 시장 기회와 향후 동향
ksm 24.09.20The Preclinical CRO Market size is estimated at USD 7.10 billion in 2024, and is expected to reach USD 10.10 billion by 2029, growing at a CAGR of 7.44% during the forecast period (2024-2029).
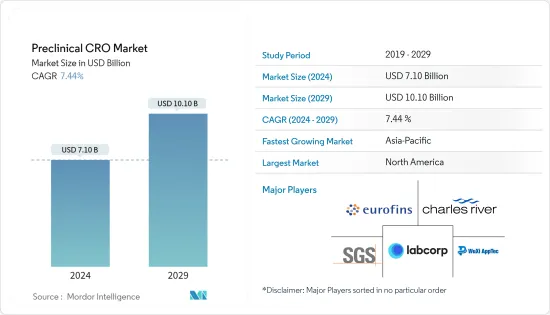
The studied market growth is primarily attributed to increasing research and development (R&D) expenditure worldwide, increasing the number of drugs in preclinical trials, and high demand for medicines uptake by chronically ill patients between 2024 and 2029.
The increasing research and development expenditure in life sciences and substantial public and private funding spending in the sector boosts market growth. For instance, the Indian Ministry of Science and Technology allocated a budget of INR 40 billion (USD 427.20 million) for the Department of Biotechnology (DBT) of India in the Union Budget for the year 2023-2024. The significant increase in funding is primarily due to the increasing R&D expenditure in the country. Hence, the rising research and development expenditure is expected to increase the adoption of preclinical CRO services to decrease the overall cost of the drug development process.
Similarly, the European Federation of Pharmaceutical Industries and Association (EFPIA) pharmaceutical research and development expenditure in 2022 reached USD 44 billion, which grew by 4.6% compared to the 2021 expenditure. Hence, the increasing R&D expenditure in Europe is expected to increase the demand for preclinical CRO services to reduce the expenditure from 2024 to 2029.
Additionally, the increasing number of drugs in preclinical stages is also expected to increase the demand for outsourcing preclinical services to reduce the cost of development and increase the chances of drug approval for conducting clinical trials. For instance, according to the ClinicalTrials.gov 2024 updated data, as of January 2024, nearly 479 thousand clinical studies were registered globally. This significant increase in clinical trials reflects the number of therapeutics and medical devices that have undergone preclinical trials and received New Drug Application Approval (NDA). Hence, the increasing number of drugs in the preclinical trials is expected to drive the studied market between 2024 and 2029.
However, a lack of standardization, monitoring issues, and stringent regulatory policies are expected to hamper the market's growth.
Preclinical CRO Market Trends
The Toxicology Testing Segment is Predicted to Witness Significant Growth Between 2024 and 2029
Toxicology testing involves the evaluation of the potential adverse effects of chemical substances or pharmaceutical compounds on living organisms. It encompasses a range of methodologies to assess these substances' safety profile, including their potential to cause harm or toxicity. In the dynamic landscape of pharmaceutical research and development, the demand for preclinical toxicology testing services offered by contract research organizations (CROs) is witnessing a significant upsurge. This surge can be attributed to various factors, including the increasing collaboration between pharmaceutical companies and CROs and technological advancements enhancing the efficiency and accuracy of toxicology testing processes.
The expanding partnership between pharmaceutical companies and CROs drives the demand for toxicology testing services. As pharmaceutical companies intensify their drug development efforts, they seek CROs' expertise and infrastructure to conduct essential preclinical toxicology studies. For instance, in May 2023, Immuter partnered with Charles River Laboratories to conduct a good laboratory practice (GLP) toxicology study for its proprietary candidate IMP761 to address autoimmune diseases. Similarly, in March 2024, Badvus Research forged a strategic alliance with Southern Research, focusing on preclinical toxicology testing and regulatory approval processes. These collaborations underscore the pivotal role of CROs in supporting drug development initiatives and ensuring regulatory compliance through comprehensive toxicology assessments.
Toxicology testing in preclinical CROs serves as a vital component of the drug development process, enabling pharmaceutical companies to evaluate the safety profiles of their candidates and make informed decisions regarding their progression in the development pipeline. Hence, increasing the vertical collaboration between pharma companies and CROs is likely to enhance the segmental growth of the market.
North America is Expected to Hold a Significant Share of the Market Between 2024 and 2029
The North American preclinical CRO market is expected to benefit from higher in-house drug development and discovery costs, rising prevalence of chronic diseases, increasing complexity in clinical trials, and a high number of investigational candidates in the development pipeline.
The higher prevalence of chronic diseases like cardiovascular diseases, diabetes, cancer, and neurological disorders necessitates the development of new therapies and treatments. Such a high and evolving burden of chronic diseases forces pharma companies to outsource preclinical trials to investigate promising candidates against those diseases, which is projected to foster the country's market growth over the study period. For instance, according to the American Cancer Society, the prevalence of lung cancer is increasing in the country, and the country reported 238,340 lung cancer cases in 2023 compared to 236,740 in 2022. This data shows a rapid increase in the incidence of cancer cases in the country, and between 2024 and 2029, the incidence of cancer will further increase; thus, the escalating burden of cancer cases in the country is projected to spur the demand for advanced therapeutics and which is in turn projected to foster preclinical trial activities and is anticipated to support country's market growth over the study period.
In addition, the higher in-house drug discovery and development costs facilitate pharmaceutical companies to outsource preclinical trial activities to reduce the cost burden and increased complexity associated with clinical trials. Thus, drug discovery and development costs are anticipated to foster demand for preclinical contract research organizations (CROs) to conduct clinical trials as they have all the required infrastructures. For instance, according to a study conducted by the World Health Organization (WHO) in 2022, the average cost to develop a new drug ranges from USD 43.4 million to USD 4.2 million. Thus, the significant cost associated with new drug development is expected to facilitate the demand for preclinical CROs to perform clinical trials and other research activities on behalf of pharmaceutical companies, as CROs have a better experience and infrastructure to conduct research services. Thus, the high demand for preclinical CROs to conduct research activities is projected to support the country's market growth over the study period.
Hence, the high prevalence of chronic diseases and the higher in-house drug discovery and development costs are expected to drive the studied market in North America between 2024 and 2029.
Preclinical CRO Industry Overview
The preclinical CRO market is highly competitive and fragmented in nature, with the presence of various well-known service providers globally. Major market players compete against each other by expanding their range of solutions and services. There are a few companies that are currently dominating the market. Key players have been involved in various strategic alliances such as acquisitions, collaborations, and the launch of advanced services to secure their position in the global market. These companies include Eurofins Scientific, Charles River Laboratories, WuXi Apptec, Labcorp Drug Development, and SGS SA.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Research and Development (R&D) Expenditure Worldwide
- 4.2.2 Increase in Number of Drugs in Preclinical Trials
- 4.2.3 High Demand for Medicines Uptake by Chronically Ill Patients
- 4.3 Market Restraints
- 4.3.1 Lack of Standardization and Monitoring Issue
- 4.3.2 Stringent Regulatory Policies
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Service
- 5.1.1 Toxicology Testing
- 5.1.2 Bioanalysis and Drug Metabolism and Pharmacokinetics Studies
- 5.1.3 Safety Pharmacology
- 5.1.4 Other Services
- 5.2 By Mode Type
- 5.2.1 Patient Derived Organoid (PDO) Models
- 5.2.2 Patient Derived Xenograft (PDX) Models
- 5.3 By End Users
- 5.3.1 Biopharmaceutical Companies
- 5.3.2 Research Institutes and Universities
- 5.3.3 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Charles River Laboratories
- 6.1.2 Labcorp Drug Development
- 6.1.3 Thermo Fisher Scientific Inc. (Pharmaceutical Product Development (PPD))
- 6.1.4 NorthEast BioAnalytical Laboratories LLC
- 6.1.5 Parexel International Corporation
- 6.1.6 Medpace
- 6.1.7 Eurofins Scientific
- 6.1.8 WuXi App Tec
- 6.1.9 ICON PLC
- 6.1.10 SGS SA
- 6.1.11 PharmaLegacy Laboratories
- 6.1.12 Altasciences Company Inc.

















