
|
시장보고서
상품코드
1685691
요로감염증(UTI) 치료제 시장 : 시장 점유율 분석, 산업 동향 및 통계, 성장 예측(2025-2030년)Urinary Tract Infection Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
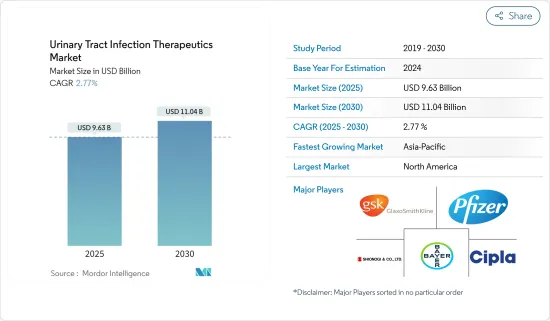
요로감염증 치료제 시장 규모는 2025년에 96억 3,000만 달러로 추정되고, 예측 기간인 2025-2030년 CAGR 2.77%로 성장할 전망이며, 2030년에는 110억 4,000만 달러에 달할 것으로 예측됩니다.

코로나바이러스 감염 환자의 전례 없는 증가는 다른 1차 진료 서비스에 대한 접근을 감소시키고 요로감염증과 같은 COVID-19와 관련이 없는 진단의 상당한 감소를 초래하였습니다. 2020년 요로감염증 진단의 주간 비율은 약간 감소했고, 이는 지난 몇 개월 동안 업계의 성장을 둔화시켰을 가능성이 있습니다. 2020년 12월에 ADIAN Journal에 게재된 연구에 따르면 COVID-19의 유행으로 인한 요로감염증(UTI) 진단의 상당한 감소가 우려됩니다. COVID-19 감염자가 SARS-CoV-2에 감염되었을 때, 팬데믹은 1차 진료를 극적으로 바꿨습니다. 그 결과, 1차 케어 서비스를 요구하는 환자가 감소하였고, 요로결석을 포함한 진단도 감소했습니다. 2020년 3월 30일에서 4월 24일 사이에 인구 10만 명당 요로결석 진단의 주간 비율은 영국의 평균 30-35에서 10 미만으로 감소했습니다. 4월 이후 정상 50%의 비율로 상승하고 있습니다. 요로결석 진단률의 상승은 시장 성장에 긍정적인 영향을 미칩니다. 시장 성장을 가속하는 요인으로는 당뇨병이나 신장 결석의 유병률 증가, 배합제의 발매 등이 있습니다.
미국 가정의학회(AAFP)를 통해, 2020년 3월 레오나르도 페레이라의 연구에 따르면 신장 결석은 일반적인 질병이며 연간 발생률은 1,000명당 8건씩 발생하는 흔한 질병이었습니다. 이 출처에 따르면 남성의 약 13%, 여성의 약 7%가 평생 동안 신장 결석을 일으킬 수 있으며, 미국의 요폐의 총 발생률은 남성 1,000명당 연간 4.5-6.8명이었습니다. 또한 국제 당뇨병 연합이 2021년에 발표한 보고서에 따르면 2020년 당뇨병에 걸린 20-79세의 사람은 세계에서 약 4억 6,300만 명이었습니다. 이 수는 2030년에는 6억 4,300만 명, 2045년에는 7억 명으로 증가할 것으로 예측됩니다. 대부분의 국가에서 2형 당뇨병 환자의 비율이 증가하고 있으며, 중저소득국에서는 성인의 79%가 당뇨병에 걸려 있습니다. 요로 감염은 소변의 당분이 박테리아의 온상이 되기 때문에 당뇨병 환자에게 특히 성가시게 됩니다. 따라서 당뇨병과 신결석의 유병률이 증가함에 따라 요로감염증(UTI)의 증례 수가 증가하고 약물 수요가 증가하기 때문에 세계의 요로감염증 치료제 시장을 견인하고 있습니다.
게다가 보다 효율적인 배합제의 발매와 노인 인구 증가가 요로감염증 치료제 시장의 성장을 뒷받침할 것으로 예상되고 있습니다. 예를 들어 프랑스 제약회사 Allecra는 2020년 2월 신규 스펙트럼 연장형 베타락타마제 억제제인 엔메타조박탐과 4세대 세팔로스포린인 세페핌의 배합제 Exblifep을 발표하고 복잡성 요로 감염을 대상으로 한 임상시험에서 주요 평가 항목을 달성했습니다.
그러나 약물 사용과 관련된 부작용과 신흥 국가 및 저개발 국가의 요로결석 유병률에 대한 인식 부족은 시장 성장을 억제합니다.
요로감염증(UTI) 치료제 시장 동향
요로감염증 치료제 시장에서는 복잡성 요로결석 부문이 큰 점유율을 차지할 전망
약물 내성 박테리아 증가 및 항생제의 과도한 사용으로 인해 복잡성 요로결석 유병률은 앞으로 증가할 것으로 예상됩니다. 대다수 의사들은 복잡성 요로결석 사례를 치료하기 위해 퀴놀론 항균제를 처방합니다. 세팔로스포린은 복잡성 요로 결석 사례에 처방되는 두 번째로 많은 약물입니다.
2020년에 International Journal of Molecular medicine에 게재된 논문에 따르면, 신결석증은 신결석증 또는 요로결석증이라고도 불리며 의학상 가장 오래된 질환 중 하나입니다. 11%의 사람들이 평생 어딘가에서 신결석을 발병하는 것으로 추정되고 있으며, 신결석의 유병률과 발병률은 세계적으로 증가하고 있습니다. 그러나 요로결석의 전반적인 유병률은 11.2%로 조사 대상자의 48.8%가 일단 친족 중에 이 질병을 가진 사람이 있었습니다. 남성은 여성보다 1.8배 요석증을 앓을 가능성이 높았습니다. 전반적으로 복잡성 요로결석의 유병률은 예측 기간 동안 증가하는 것으로 보입니다.
그러나 미국 식품의약국에서 승인 증가와 주요 기업의 제품 출시가 시장을 밀어올릴 것으로 예상됩니다. 예를 들어, 2019년 7월 미국 식품의약국은 성인의 복잡성 요로 감염(cUTI) 및 복잡한 복강내 감염(cIAI)을 치료하기 위한 항균제 제품인 Merck and Company의 Recarbrio(이미페넴, 실라스타틴 및 레리박탐)를 승인했습니다.
이와 같이 cUTI 유병률 상승 및 제품 출시 증가와 같은 전술한 요인은 모두 예측 기간 중에 이 부문을 밀어올릴 것으로 예상됩니다.
북미가 예측 기간 동안 큰 시장 점유율을 차지할 전망
이 지역에서는 요로결석에 사용되는 진단 방법에 관한 기술 혁신이 급격히 증가하고 있습니다. 2020년 11월에 Infectious Diseases Society of America에 게재된 Pranita D. Tamma의 논문에 따르면 요로결석의 정기 검진 중에 한 여성이 최후의 보루인 항생제 콜리스틴에 내성을 나타내는 대장균에 감염된 것이 관찰되었습니다. 콜리스틴 내성균의 발견은 큰 문제가 되었습니다. 또한 CDC는 다른 조직과 공동으로 미국에서 카테터 관련 요로결석 및 기타 건강 관리 관련 감염을 예방하기 위한 지침을 만들었습니다. 또한 Queensland Pediatric Factsheet 2019는 7세까지 여아의 약 10명 중 1명, 남아의 약 50명 중 1명이 요로감염을 앓고 있다고 추정합니다. 1세 미만의 감염은 남아에게 많지만, 연장아에서는 여아에게 많습니다.
게다가 2019년 5월에 'Therapeutic Advances in Urology'에 게재된 '요로감염의 역학 및 부담에 대한 소개'라는 제목의 연구에 따르면 요로감염증(UTI)은 성인 여성의 외래 감염증에서 가장 많고 평생 유병률은 50-60%입니다. 요로감염증은 사회적으로나 개인적으로 큰 부담이 되고 있으며, 미국에서는 매년 상당한 수의 진찰이 요로감염증에 의해 점유되고 있습니다. 이 질환은 요로감염증 치료제의 판매를 증가시키고 시장을 견인할 것으로 예상됩니다.
또한, 요로감염증을 위한 새로운 유형의 항생제 연구 및 개발은 시장 성장을 도울 것으로 예상됩니다. 예를 들어, 2020년 8월에는 캘리포니아 공과 대학의 연구자들이 박테리아의 철 획득을 목표로 하는 새로운 유형의 요로감염증을 위한 항생제 개발을 발표했습니다.
이와 같이 앞서 언급한 모든 요인은 예측기간 동안 이 지역 시장을 밀어올릴 것으로 예상됩니다.
요로감염증 치료제 산업 개요
요로감염증 치료제 시장은 세분화된 경쟁 시장이며, 수많은 선도 기업들로 구성되어 있습니다. 현재 시장을 독점하고 있는 기업으로는 AstraZeneca, Bayer AG, Cipla Inc., GlaxoSmithKline PLC, Shionogi & Co. Ltd, Novartis AG, 및 Pfizer 등이 있습니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사 전제조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 역학
- 시장 개요
- 시장 성장 촉진요인
- 당뇨병 및 신결석 증가
- 배합제의 발매
- 시장 성장 억제요인
- 약의 사용에 따른 부작용
- 신흥 국가 및 저개발 국가에서의 인식 부족
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자 및 소비자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업간 경쟁 관계의 강도
제5장 시장 세분화
- 의약품별
- 페니실린 및 그 배합제
- 퀴놀론계 항균제
- 세팔로스포린
- 아졸 및 암포테리신 B
- 니트로푸란
- 기타 의약품(아미노글리코시드 항체, 술폰아미드, 테트라사이클린 등)
- 적응증별
- 합병증성 요로결석
- 합병증이 없는 요로결석
- 기타 적응증(합병증을 반복하는 요로 결석, 신경인성 방광 감염증 등)
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 기업 프로파일
- AstraZeneca
- Bayer AG
- Cipla Inc.
- GlaxoSmithKline PLC
- Shionogi & Co. Ltd
- Novartis AG
- Pfizer
- Merck & Co. Inc
- Bristol-Myers Squibb Company
- Almirall SA
- Dr. Reddys Laboratories Ltd
- Allergan
제7장 시장 기회 및 향후 동향
AJY 25.04.17The Urinary Tract Infection Therapeutics Market size is estimated at USD 9.63 billion in 2025, and is expected to reach USD 11.04 billion by 2030, at a CAGR of 2.77% during the forecast period (2025-2030).
The unprecedented increase in coronavirus-infected patients had reduced access to other primary care services and resulted in a significant drop in non-COVID-19-related diagnoses, such as urinary tract infection. The weekly rate of UTI diagnosis fell slightly in 2020, which may have slowed the industry's growth in recent months. According to a study published in the ADIAN Journal in December 2020, a significant decrease in urinary tract infection (UTI) diagnoses due to the COVID-19 pandemic raises concern. When people with COVID-19 were infected with SARS-CoV-2, the pandemic changed primary care dramatically. This resulted in fewer patients seeking primary care services and fewer diagnoses, including UTIs. Between March 30 and April 24, 2020, the weekly rate of UTI diagnosis per 100,000 population fell from an average of 30-35 to less than 10 in England. Since April, the rate has risen by 50% of the usual rate. The increase in the rate of UTI diagnosis has positively impacted market growth. Certain factors driving the market growth include the increasing prevalence of diabetes and kidney stones and the launch of combination drugs.
According to the American Academy of Family Physicians (AAFP), in March 2020, by Leonardo Ferreira, kidney stones were a common ailment, with an annual incidence of eight instances per 1,000 persons. As per the same source, around 13% of men and 7% of women may develop a kidney stone during their lifetime, and the total incidence of urinary retention in the US is 4.5 to 6.8 per 1,000 men per year. Moreover, according to the International Federation of Diabetics report in 2021, diabetes affected approximately 463 million persons aged 20 to 79 years worldwide in 2020. This number is anticipated to climb to 643 million by 2030 and 700 million by 2045. In most countries, the proportion of people with Type 2 diabetes is increasing, and diabetes affects 79% of adults in low- and middle-income countries. Urinary tract infections can be especially troublesome for people with diabetes because sugar in the urine serves as a breeding ground for bacteria. Therefore, with the increasing prevalence of diabetes and kidney stones, the number of cases of urinary tract infection (UTI) increases, increasing the demand for drugs, thus, driving the global urinary tract infection therapeutics market.
Additionally, the launch of more efficient combination drugs and the increasing geriatric population is expected to boost the growth of the urinary tract infection therapeutics market. For instance, in February 2020, Allecra, a French pharmaceutical company, announced Exblifep, a combination of enmetazobactam, a novel extended-spectrum beta-lactamase inhibitor, and cefepime, a fourth-generation cephalosporin that met primary endpoints in a clinical trial for complicated UTIs.
However, adverse events associated with the use of medication and the lack of awareness about the prevalence of UTIs in developing and underdeveloped countries are restraining the market's growth.
Urinary Tract Infection (UTI) Therapeutics Market Trends
Complicated UTIs Segment Expected to Hold a Major Share in the Urinary Tract Infection Therapeutics Market
The prevalence of complicated UTIs is expected to increase in the future, owing to the rise in drug-resistant bacteria and excessive use of antibiotics. A vast majority of physicians prescribe quinolones to treat complicated UTI cases. Cephalosporin is the second-most common drug prescribed for complicated UTI cases.
According to an article published in the International Journal of Molecular medicine in 2020, kidney stone disease, also known as nephrolithiasis or urolithiasis, is one of medicine's oldest diseases. It is estimated that 11% of people will develop kidney stones at some point in their lives, and the prevalence and incidence of kidney stones are increasing worldwide. However, the overall prevalence of urolithiasis was 11.2%, with 48.8% of those surveyed having a first-degree relative with the disease. Males were 1.8 times more likely than females to have urolithiasis. Overall, the prevalence of complicated UTIs is set to increase during the forecast period, mainly owing to the increasing bacterial resistance in UTI cases and the rise in recurrence rate for UTIs.
However, increasing approvals from the US Food and Drug Administration and product launches by key players are expected to boost the market. For instance, in July 2019, the US Food and Drug Administration approved Merck and Company's Recarbrio (imipenem, cilastatin, and relebactam), an antibacterial drug product to treat adults with complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI).
Thus, all aforementioned factors such as rising prevalence of cUTIs and increasing product launches are expected to boost the segment over the forecast period.
North America Expected to Hold Significant Market Share in the Forecast Period
The region is experiencing a drastic increase in innovations related to diagnostic methodologies used for UTIs. According to the article published in Infectious Diseases Society of America in November 2020, by Pranita D. Tamma, during a routine checkup for a UTI, a woman was observed to be infected by E. coli that showed resistance to the last-resort antibiotic, Colistin. The discovery of Colistin-resistant bacteria was considered a major issue. In addition to that, the CDC, in collaboration with other organizations, developed guidelines for preventing catheter-associated UTIs and other healthcare-associated infections in the US. Furthermore, the Queensland Pediatric Factsheet 2019 estimated that approximately 1 in 10 girls and 1 in 50 boys would suffer from a urinary tract infection by seven years of age. Infections in children under one year are more common in boys, but in older children, infections are more common in girls.
Additionally, according to a study published in Therapeutic Advances in Urology in May 2019 titled, "An Introduction to the Epidemiology and Burden of Urinary Tract Infections," urinary tract infections (UTIs) are the most common outpatient infections in adult women, with a lifetime prevalence of 50-60%. UTIs are a substantial societal and personal burden, with UTIs accounting for a significant number of medical visits in the US each year. This condition is expected to drive the market by increasing the sales of urinary tract infection medicines.
Furthermore, research and development of novel classes of antibiotics for urinary tract infections are expected to aid in market growth. In August 2020, for example, researchers at California Polytechnic State University announced the development of a new class of antibiotics for urinary tract infections that target bacterial iron acquisition.
Thus, all aforementioned factors are expected to boost the market in the region over the forecast period.
Urinary Tract Infection (UTI) Therapeutics Industry Overview
The urinary tract infection therapeutics market is fragmented and competitive and consists of a number of major players. Some of the companies currently dominating the market are AstraZeneca, Bayer AG, Cipla Inc., GlaxoSmithKline PLC, Shionogi & Co. Ltd, Novartis AG, and Pfizer, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Diabetes and Kidney Stones
- 4.2.2 Launch of Combination Drugs
- 4.3 Market Restraints
- 4.3.1 Adverse Effects Associated with the Use of Medication
- 4.3.2 Lack of Awareness in Developing and Underdeveloped Countries
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 Drug
- 5.1.1 Penicillin and Combinations
- 5.1.2 Quinolones
- 5.1.3 Cephalosporin
- 5.1.4 Azoles and Amphotericin B
- 5.1.5 Nitrofurans
- 5.1.6 Other Drugs (Aminoglycoside Antibodies, Sulphonamides, Tetracycline, etc.)
- 5.2 Indication
- 5.2.1 Complicated UTI
- 5.2.2 Uncomplicated UTI
- 5.2.3 Other Indications (Recurring Complicated UTI, Neurogenic Bladder Infection, etc.)
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 US
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 UK
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle-East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle-East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 AstraZeneca
- 6.1.2 Bayer AG
- 6.1.3 Cipla Inc.
- 6.1.4 GlaxoSmithKline PLC
- 6.1.5 Shionogi & Co. Ltd
- 6.1.6 Novartis AG
- 6.1.7 Pfizer
- 6.1.8 Merck & Co. Inc
- 6.1.9 Bristol-Myers Squibb Company
- 6.1.10 Almirall SA
- 6.1.11 Dr. Reddys Laboratories Ltd
- 6.1.12 Allergan

















