
|
시장보고서
상품코드
1852095
RWE(Real World Evidence) 솔루션 시장 : 시장 점유율 분석, 산업 동향, 통계, 성장 예측(2025-2030년)Real-World Evidence Solutions - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
RWE(Real World Evidence) 솔루션 시장 규모는 2025년에 24억 4,000만 달러로 추정되고, 2030년에는 52억 1,000만 달러에 이를 것으로 예측되며, CAGR 16.53%로 성장할 전망입니다.

디지털화된 임상 데이터, 유전체 데이터, 관리 데이터세트는 주요 의료 시스템 전체에서 2자리 속도로 확대되고 있으며, 미국, 유럽 연합, 일본의 규제 당국은 스폰서가 과학적 엄밀성을 희생하지 않고 개발 기간을 단축하며 기존과 다른 데이터를 신청서에 통합하는 방법에 대한 지침을 발표하고 있습니다. 바이오파마의 예산은 채용 리스크를 저감하는 대규모 환자 코호트에 기울고 있으며, 지불자는 프리미엄 가격을 결과와 연결하고 있기 때문에 제조업체는 출시 시에 실세계에서의 유효성을 검증하는 애널리틱스를 채용할 수밖에 없게 되고 있습니다. 벤처 캐피탈로부터의 자금 유입은 확장 가능한 클라우드 아키텍처를 갖춘 플랫폼 기업에게 유리하며, 틈새 데이터 세트를 획득하고 공유를 통합하기 위한 자금을 제공합니다. 동시에 토큰화 및 연계 학습 등의 프라이버시 보호 기술이 조달의 전제조건이 되고 있어 입증된 보안과 거버넌스를 가진 벤더로 계약이 유도되고 있습니다.
세계의 RWE(Real World Evidence) 솔루션 시장 동향 및 인사이트
주요 기관에서 높아지는 규제 당국의 승인
미국 FDA의 RWE 프레임워크 및 해당 파일럿 프로그램은 보험 청구 및 EHR 기록에서 구축된 외부 관리 코호트를 제출하기 위한 공식적인 경로를 수립했습니다. 유럽 의약품청(EEA)은 데이터 분석과 실임상시험 네트워크(Data Analysis and Real-World Interrogation Network) 하에서 이 동향을 반영하여 여러 합성 항암제 제안에 대해 긍정적인 적격성 의견을 발표하고 있습니다. 일본의 의약품 의료기기 종합기구(PMDA)도 2024년 실 데이터 신뢰성 시험에 관한 지침을 발표하고 있습니다. 스폰서는 현재 2단계 초기에 관찰 엔드포인트를 통합하여 매우 중요한 임상시험에서 불확실성을 줄이고 있습니다. 이와 같이 투명성이 높은 데이터 연계는 컴플라이언스의 뒷받침에서 최전선 차별화 요인으로 변화하여 감사 대응 가능한 파이프라인을 제공하는 벤더에게 보상을 주고 위험을 싫어하는 바이오의약품 조달팀의 계약 사인오프를 가속화하고 있습니다.
디지털화된 의료 데이터의 급속한 확장
미국의 비연방 급성기 병원의 전자 차트 도입률은 2024년에 89.0%를 넘었으며, RWE(Real World Evidence) 솔루션 시장에 페타바이트급의 구조화 데이터가 추가됩니다. 웨어러블은 지속적인 생리적 스트림을 생성하고 차세대 시퀀서의 출력은 분자 서명으로 질병 등록을 향상시킵니다. 멀티모달 링크를 통해 연구자들은 이미지, 약국 청구, 사회적 결정 지표를 결합할 수 있어 전통적인 임상시험에서 보이지 않은 표현형을 밝힐 수 있습니다. 그러나 EU의 GDPR(EU 개인정보보호규정)과 캘리포니아의 CPRA와 같은 엄격한 개인정보 보호법은 감시를 강화하고 있습니다. 식별자를 돌이킬 수 없는 해시로 변환하는 토큰화 공급자가 중심 파트너가 되어 원시 파일을 집계하는 대신 코드를 데이터로 이동시키는 연합 학습 네트워크는 거주 규칙을 위반하지 않고 국경을 넘어서는 협업을 가능하게 합니다. 공통 데이터 모델 하에서 이기종 분류법을 조화시킬 수 있는 공급업체는 연구 시작을 수개월 단축하고 측정 가능한 이점을 얻습니다.
실행 가능한 인사이트를 추출하기 위한 성숙한 AI 및 고급 분석 플랫폼
Transformer 기반 자연언어 처리 모델은 2024년 검증 연구에 있어서, 비구조화 병리 보고서에서 종양학 종점을 추출하여 0.90 이상의 F1 점수를 달성하여 수동 추출 비용을 60% 이상 절감했습니다. IQVIA의 어플라이드 AI 포트폴리오를 통해 도입된 NVIDIA의 DGX H100 클러스터는 모델의 훈련 시간을 며칠에서 몇 시간으로 단축하여 예측 모델의 신속한 반복을 가능하게 합니다. 합성 데이터 생성 기술은 클래스 불균형과 개인 정보 보호 제약을 해결하고 식별 가능한 레코드를 노출하지 않고 교육 세트를 확장합니다. 이러한 생산성 향상은 프리미엄 라이선스 비용을 정당화하고 AI 플랫폼의 성장을 RWE(Real World Evidence) 솔루션 시장 전체보다 가속화합니다. 또한 GPU의 빠른 추론은 쿼리 대기 시간을 줄여줍니다. 이는 지불자와 협상하는 동안 온디맨드로 증거 검색을 수행하는 의료용 팀에게 중요한 구매 기준이 됩니다.
부문 분석
2024년 RWE(Real World Evidence) 솔루션 시장의 55.0%는 서비스에 의한 것으로, 스폰서가 시험 설계, 데이터 큐레이션, 규제 전략에 있어서 외부의 역학자, HEOR 컨설턴트, 생물통계학자에 의존하고 있음을 반영하고 있습니다. IQVIA, ICON, Syneos Health와 같은 선도적인 서비스 제공업체는 약국 청구와 EHR 피드를 연결하는 토크나이제이션 파이프라인을 번들로 연결하여 종단 추적 조사를 확대하고 고객의 스위칭 비용을 높입니다. 다년간의 아웃소싱 틀은 예측 가능한 수익의 가시성을 보장하고 거시 경제의 변동을 완화합니다. 서비스팀은 또한 GDPR(EU 개인정보보호규정) 하에서 요구되는 프라이버시 영향 평가에 대해 조언하고 유럽의 시험 승인을 가속화하고 있습니다.
소프트웨어는 플랫폼 공급업체가 클라우드 네이티브 아키텍처를 상용화함에 따라 현재 규모가 작지만, CAGR 18.0%로 확대되고 있습니다. 구독 모델은 불안정한 프로젝트 비용을 대체하여 공급업체의 현금 흐름을 개선합니다. 코어 플랫폼에 내장된 AI 모듈은 방사선 검사 및 유전체 보고서에서 엔드포인트를 자동으로 추출하여 수동 코딩 병목 현상을 제거합니다. 예를 들어 ConcertAI의 SaaS 제품군은 비구조화된 병리 메모를 캡처하고, 변환 모델에서 종양의 병기 분류를 수행하며, 분석에 적합한 구조화된 데이터 형식을 반환합니다. 플랫폼 채택은 종종 맞춤형 애널리틱스에 대한 후속 서비스 요청을 유도하고 소프트웨어 부서와 컨설팅 부서 사이에 공생 성장 루프를 생성합니다.
클라우드는 2024년 RWE(Real World Evidence) 솔루션 시장 규모의 65.0%를 차지했으며, 탄력적 컴퓨팅 및 종량 과금제의 혜택을 누리고 있습니다. RWE 애널리틱스의 AWS 마켓플레이스 출품은 전년 대비 40% 이상 증가하여 공유 책임 보안 모델을 충족하는 사전 승인 공급업체에 대한 구매자의 강한 선호를 보여줍니다. 초기 마이그레이션에는 비식별화된 코호트가 포함되며, 보호된 의료 정보는 암호화 프레임워크와 키 관리 정책이 성숙된 후에 마이그레이션됩니다. 미국의 의료 시스템은 피크 시에 퍼블릭 클라우드의 GPU 버스트를 활용하여 NLP 모델을 교육하고 자본 집약적인 서버 구매를 피하고 있습니다.
하이브리드의 도입이 CAGR 21.0%로 진행되고 있어, 학술 의료 센터나 공적 자금에 의한 연구 네트워크가, 온프레미스의 데이터 주권 및 스케일러블한 애널리틱스의 밸런스를 취하고 있습니다. 예를 들어, Oracle의 Cloud@Customer 노드는 병원 방화벽 안에 있는 동안 유럽 데이터 보호위원회(European Data Protection Board)의 레지던시 지침을 충족하는 공용 리전과 연동하여 고부하 컴퓨팅 작업을 수행할 수 있습니다. 정책 기반 워크로드 오케스트레이션을 제공하는 공급업체는 PHI를 고려한 쿼리를 자동으로 개인 클러스터로 라우팅합니다. 자본 집약적 인사이트는 기존 서버 랙의 수명을 연장하고 동시에 버스트 워크로드를 위해 클라우드 GPU에 액세스하여 총 소유 비용을 향상시킵니다.
지역 분석
2024년의 RWE(Real World Evidence) 솔루션 시장은 북미가 41.3%의 점유율로 리드했습니다. FDA의 RWE 파일럿 프로그램은 명확한 절차 지침을 제공하고 스폰서의 증거 위험을 줄이는 반면, 미국 보험 회사는 고액 약제 계약에 결과 측정을 통합하고 간접적으로 컴플라이언스를 준수하는 분석 수요를 촉진하고 있습니다. 자본 시장은 데이터 중심의 비즈니스 모델을 평가하고 나스닥에 상장된 RWE 공급업체의 가치는 임상 CRO의 동업자를 능가하는 수익 배율로 거래되고 있으며 제품 로드맵에 적극적인 재투자를 가능하게 합니다.
유럽은 국경을 넘은 데이터 재이용을 위한 기술적 및 법적 틀을 의무화하는 유럽 의료 데이터 스페이스 규제 시행에 힘입어 2위를 차지했습니다. GDPR(EU 개인정보보호규정)을 준수하는 아키텍처와 HDS 인증을 통해 공급업체는 각국의 의료 서비스와의 연계를 원활하게 합니다. 다수의 부담 환경은 틈새 기회를 촉진합니다. : 프랑스의 ATU 시스템과 독일의 AMNOG 패스웨이는 추가적인 이익을 확인하기 위해 실세계의 증거를 받아들이게 되어, 특수한 종양학 및 희귀질환의 데이터 세트에 비즈니스 기회를 가져오고 있습니다.
아시아태평양은 CAGR 17.8%로 예측되는 급성장 지역입니다. 중국의 국가약품감독관리국은 2024년 신약 추가 신청에 있어서 외국의 리얼 월드 데이터의 수락에 관한 가이던스를 발표하고 다국적 스폰서의 신청 장벽을 인하했습니다. 일본의 후생노동성이 디지털 바이오마커 시험 연구에 자금을 제공해 신경학 연구의 정보원을 확대합니다. 호주 마이헬스 레코드 시스템은 인구 커버리지 95%를 넘어 해외 스폰서를 유치하는 견고한 종단 데이터 세트를 만듭니다. 국경을 넘는 관민 파트너십은 데이터 사전을 표준화하여 여러 국가의 코호트 풀링을 가능하게 하여 세계 AI 모델의 알고리즘 범용성을 향상시키고 있습니다.
기타 혜택 :
- 엑셀 형식 시장 예측(ME) 시트
- 3개월간의 애널리스트 서포트
목차
제1장 서론
- 조사의 전제조건 및 시장 정의
- 조사 범위
제2장 조사 방법
제3장 주요 요약
제4장 시장 상황
- 시장 개요
- 시장 성장 촉진요인
- 주요기관에 있어서 규제의 수용성
- 디지털화된 헬스케어 데이터의 확대
- 외부 대조군의 의약품 이용
- 가치 기반의 상환 모델
- 성숙하는 인공지능 및 고도 분석 플랫폼
- 크로스, 기술 벤더, 의료 시스템 간의 전략적 콜라보레이션
- 시장 성장 억제요인
- 데이터 프라이버시 및 상호 운용성의 장애물
- 크로스보더 연구에 있어서 규제의 분단
- 큐레이션된 종단 데이터 세트의 높은 취득 비용 및 라이선스 비용
- RWE 조사 방법적 엄밀성 및 바이어스에 관한 이해 관계자의 회의론
- Porter's Five Forces 분석
- 신규 참가업체의 위협
- 구매자의 협상력
- 공급기업의 협상력
- 대체품의 위협
- 경쟁 기업 간 경쟁 관계
제5장 시장 규모 및 성장 예측
- 컴포넌트별
- 서비스
- 데이터 세트
- 임상 설정 데이터
- 청구 데이터
- 약국 조제 데이터
- 환자 주도 데이터 및 PRO 데이터
- 기타 컴포넌트
- 소프트웨어 및 애널리틱스 플랫폼
- 전개 모드별
- 클라우드 기반
- 온프레미스
- 하이브리드
- 치유 영역별
- 종양
- 심장병학
- 당뇨병
- 신경학
- 정신의학
- 면역학
- 기타 치료 영역
- 용도별
- 의약품 개발 및 승인
- 의료기기 개발 및 승인
- 약물감시 및 안전성 시험
- 규제 당국의 결정 및 상환
- 최종 사용자별
- 의약품 및 의료기기 제조업체
- 의약품 개발 업무 수탁 기관(CRO)
- 의료기관 및 의료기관-의료기관 네트워크
- 기타 최종 사용자
- 지역별
- 북미
- 미국
- 캐나다
- 멕시코
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 기타 유럽
- 아시아태평양
- 중국
- 일본
- 인도
- 한국
- 호주
- 기타 아시아태평양
- 중동 및 아프리카
- GCC
- 남아프리카
- 기타 중동 및 아프리카
- 남미
- 브라질
- 아르헨티나
- 기타 남미
- 북미
제6장 경쟁 구도
- 시장 집중도
- 전략적 동향
- 시장 점유율 분석
- 기업 프로파일
- IQVIA Inc.
- Optum Inc.
- Oracle Health
- ICON plc
- IBM
- Syneos Health
- TriNetX LLC
- Thermo Fisher Scientific, Inc.
- Flatiron Health
- SAS Institute, Inc.
- Aetion Inc.
- Komodo Health
- Medpace Holdings Inc.
- ConcertAI
- Tempus Labs
- Clarivate
- Clinerion Ltd.
- Veeva Systems
- Verto Health
제7장 시장 기회 및 향후 전망
AJY 25.11.26The real-world evidence solutions market size stands at USD 2.44 billion in 2025 and is projected to reach USD 5.21 billion by 2030, advancing at a vigorous 16.53% CAGR.
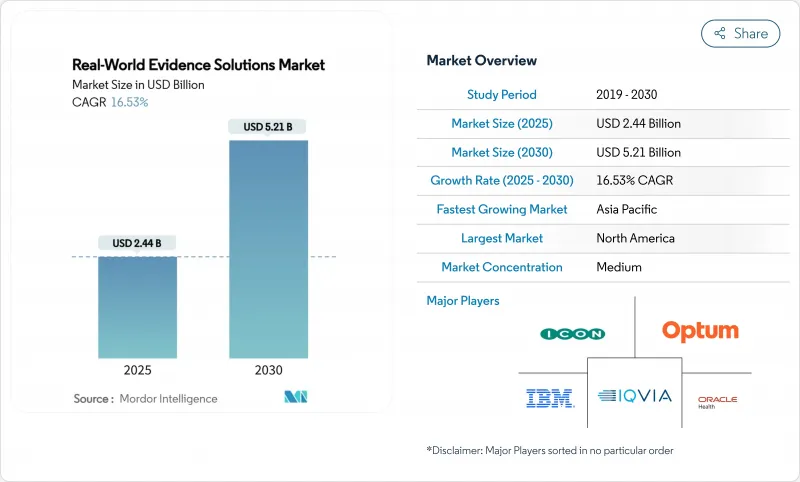
Digitized clinical, genomic and administrative data sets are expanding at double-digit rates across major healthcare systems, while regulators in the United States, European Union and Japan continue to publish guidance on how sponsors can incorporate non-traditional data into submissions, cutting development timelines without sacrificing scientific rigor. Biopharma budgets are tilting toward large, curated patient cohorts that lower recruitment risk, and payers are tying premium pricing to outcomes, forcing manufacturers to adopt analytics that validate real-world effectiveness at launch. Venture capital inflows favor platform companies with scalable cloud architectures, giving them the capital to acquire niche datasets and consolidate share. At the same time, privacy-preserving techniques such as tokenization and federated learning are becoming procurement prerequisites, steering contracts toward vendors with proven security and governance.
Global Real-World Evidence Solutions Market Trends and Insights
Growing Regulatory Acceptance Across Major Agencies
The U.S. FDA's Real-World Evidence Framework and corresponding pilot programs have formalized pathways for submitting externally controlled cohorts built from claims and EHR records. The European Medicines Agency mirrors this trend under its Data Analysis and Real-World Interrogation Network, publishing positive qualification opinions for multiple synthetic-arm proposals. Japan's PMDA followed with its 2024 guidance on real-world data reliability testing. Sponsors now embed observational endpoints as early as phase II, reducing uncertainty in pivotal trials. Transparent data lineage has thus shifted from a compliance afterthought to a frontline differentiator, rewarding vendors that deliver audit-ready pipelines and accelerating contract sign-offs among risk-averse biopharma procurement teams.
Rapid Expansion of Digitized Healthcare Data
Electronic health record adoption levels surpassed 89.0% among U.S. non-federal acute-care hospitals in 2024, adding petabytes of structured data to the real-world evidence solutions market. Wearables generate continuous physiologic streams, while next-generation sequencing outputs enrich disease registries with molecular signatures. Multi-modal linkages enable researchers to combine imaging, pharmacy claims and social-determinant indicators, uncovering phenotypes invisible to traditional trials. Yet stricter privacy statutes such as the EU's GDPR and California's CPRA are sharpening oversight. Tokenization providers that convert identifiers into non-reversible hashes have become central partners, and federated-learning networks that move code to the data rather than aggregating raw files allow cross-border collaboration without breaching residency rules. Vendors able to harmonize disparate taxonomies under common data models shorten study start-up by months, gaining a measurable edge.
AI and Advanced Analytics Platforms Mature to Extract Actionable Insights
Transformer-based natural language processing models achieved F1 scores above 0.90 on extracting oncology endpoints from unstructured pathology reports in 2024 validation studies, cutting manual abstraction costs by more than 60%. NVIDIA's DGX H100 clusters, deployed through IQVIA's Applied AI portfolio, reduce model training times from days to hours, enabling rapid iteration on predictive models. Synthetic-data generation techniques address class imbalance and privacy constraints, broadening training sets without exposing identifiable records. Such productivity gains justify premium license fees, pushing AI platform growth faster than the overall real-world evidence solutions market. GPU-accelerated inferencing also lowers query latency, a key buying criterion for medical-affairs teams conducting on-demand evidence searches during payer negotiations.
Other drivers and restraints analyzed in the detailed report include:
- Pharmaceutical Companies Leverage RWE to Curb R&D Timelines and Costs
- Value-Based Reimbursement Models Drive Outcomes-Oriented Evidence
- Data Privacy and Interoperability Challenges Hinder Seamless Integration
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Services generated 55.0% of the real-world evidence solutions market in 2024, reflecting sponsor dependence on external epidemiologists, HEOR consultants and biostatisticians for study design, data curation and regulatory strategy. Large service providers such as IQVIA, ICON and Syneos Health bundle tokenization pipelines that connect pharmacy claims with EHR feeds, extending longitudinal follow-up and raising client switching costs. Multi-year outsourcing frameworks ensure predictable revenue visibility, cushioning macro-economic swings. Services teams also advise on privacy-impact assessments required under GDPR, expediting European study approvals.
Software, though currently smaller, is scaling at an 18.0% CAGR as platform vendors commercialize cloud-native architectures. Subscription models replace volatile project fees, improving vendor cash flow. AI modules embedded in core platforms automatically extract endpoints from radiology and genomic reports, eliminating manual coding bottlenecks. ConcertAI's SaaS suite, for example, ingests unstructured pathology notes, classifies tumor staging with transformer models and returns structured data formats ready for analysis. Platform adoption often triggers follow-on service requests for bespoke analytics, creating a symbiotic growth loop between software and consulting units.
Cloud captured 65.0% of the real-world evidence solutions market size in 2024, benefiting from elastic compute and pay-as-you-go pricing. AWS Marketplace listings for RWE analytics rose by more than 40% year on year, indicating strong buyer preference for pre-approved vendors that satisfy shared-responsibility security models. Early migrations involve de-identified cohorts, with protected health information moving only after encryption frameworks and key-management policies mature. U.S. health systems leverage public cloud GPU bursts to train NLP models during peak demand, avoiding capital-intensive server purchases.
Hybrid deployment is advancing at 21.0% CAGR as academic medical centers and publicly funded research networks balance on-premise data sovereignty with scalable analytics. Oracle's Cloud@Customer nodes, for instance, sit behind hospital firewalls yet federate with public regions for high-intensity compute jobs, satisfying European Data Protection Board residency guidance. Vendors that deliver policy-based workload orchestration-automatically routing PHI-sensitive queries to private clusters-address a critical adoption hurdle and displace legacy on-prem installations. Capital-intensive sites extend the lifespan of existing server racks while accessing cloud GPUs for burst workloads, improving total cost of ownership.
The Real-World Evidence Solutions Market Report is Segmented by Component (Services, and More), Deployment Mode (Cloud-Based, and More), Therapeutic Area (Drug Development & Approvals, and More), Application (Drug Development & Approvals, and More), End User (Pharmaceutical & Medical-Device Companies, and More), and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America led the real-world evidence solutions market in 2024 with a 41.3% share. The FDA's RWE pilot programs provide clear procedural guidance, reducing evidentiary risk for sponsors, while U.S. insurers embed outcome metrics into high-cost drug contracts, indirectly driving demand for compliant analytics. Capital markets reward data-centric business models; valuations for listed RWE vendors on Nasdaq trade at revenue multiples above clinical CRO peers, enabling aggressive reinvestment in product roadmaps.
Europe ranked second, supported by the upcoming European Health Data Space regulation, which mandates technical and legal frameworks for cross-border data reuse. GDPR-compliant architectures and HDS accreditation smooth vendor onboarding with national health services. Multi-payer environments foster niche opportunities: France's ATU system and Germany's AMNOG pathway increasingly accept real-world evidence to confirm added benefit, opening business for specialized oncology and rare-disease datasets.
Asia-Pacific is the fastest-growing region, projected at a 17.8% CAGR. China's National Medical Products Administration issued 2024 guidance on accepting foreign real-world data for supplemental New Drug Applications, lowering submission barriers for multinational sponsors. Japan's MHLW funds digital-biomarker pilots, expanding sources for neurology studies. Australia's My Health Record system surpasses 95% population coverage, creating robust longitudinal datasets that attract overseas sponsors. Cross-border public-private partnerships are standardizing data dictionaries, enabling multi-country cohort pooling and improving algorithm generalizability for global AI models.
- IQVIA
- Optum
- Oracle Health
- ICON
- IBM
- Syneos Health
- TriNetX LLC
- Thermo Fisher Scientific
- Flatiron Health
- SAS Institute, Inc.
- Aetion Inc.
- Komodo Health
- MedPace
- ConcertAI
- Tempus Labs
- Clarivate
- Clinerion Ltd.
- Veeva Systems
- Verto Health
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Regulatory Acceptance Across Major Agencies
- 4.2.2 Expansion Of Digitized Healthcare Data
- 4.2.3 Pharmaceutical Use Of External Control Arms
- 4.2.4 Value-Based Reimbursement Models
- 4.2.5 Artificial-Intelligence And Advanced Analytics Platforms Maturing
- 4.2.6 Strategic Collaborations Between Cros, Tech Vendors, And Health Systems
- 4.3 Market Restraints
- 4.3.1 Data Privacy And Interoperability Hurdles
- 4.3.2 Regulatory Fragmentation In Cross-Border Studies
- 4.3.3 High Acquisition And Licensing Costs For Curated Longitudinal Datasets
- 4.3.4 Stakeholder Skepticism Regarding Methodological Rigor And Bias In RWE Studies
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Component
- 5.1.1 Services
- 5.1.2 Data Sets
- 5.1.2.1 Clinical-Settings Data
- 5.1.2.2 Claims & Billing Data
- 5.1.2.3 Pharmacy Dispensing Data
- 5.1.2.4 Patient-Powered & PRO Data
- 5.1.2.5 Other Components
- 5.1.3 Software & Analytics Platforms
- 5.2 By Deployment Mode
- 5.2.1 Cloud-based
- 5.2.2 On-premise
- 5.2.3 Hybrid
- 5.3 By Therapeutic Area
- 5.3.1 Oncology
- 5.3.2 Cardiology
- 5.3.3 Diabetes
- 5.3.4 Neurology
- 5.3.5 Psychiatry
- 5.3.6 Immunology
- 5.3.7 Other Therapeutic Areas
- 5.4 By Application
- 5.4.1 Drug Development & Approvals
- 5.4.2 Medical-Device Development & Approvals
- 5.4.3 Pharmacovigilance & Safety Studies
- 5.4.4 Regulatory Decision-making & Reimbursement
- 5.5 By End User
- 5.5.1 Pharmaceutical & Medical-Device Companies
- 5.5.2 Contract Research Organizations (CROs)
- 5.5.3 Healthcare Providers & Payer-provider networks
- 5.5.4 Other End Users
- 5.6 Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 South Korea
- 5.6.3.5 Australia
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle-East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.4.1 IQVIA Inc.
- 6.4.2 Optum Inc.
- 6.4.3 Oracle Health
- 6.4.4 ICON plc
- 6.4.5 IBM
- 6.4.6 Syneos Health
- 6.4.7 TriNetX LLC
- 6.4.8 Thermo Fisher Scientific, Inc.
- 6.4.9 Flatiron Health
- 6.4.10 SAS Institute, Inc.
- 6.4.11 Aetion Inc.
- 6.4.12 Komodo Health
- 6.4.13 Medpace Holdings Inc.
- 6.4.14 ConcertAI
- 6.4.15 Tempus Labs
- 6.4.16 Clarivate
- 6.4.17 Clinerion Ltd.
- 6.4.18 Veeva Systems
- 6.4.19 Verto Health
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment

















