
|
시장보고서
상품코드
1648562
엑소좀 요법 시장 : 치료 유형별, 적응증별, 치료 영역별, 투여 경로별, 지역별 - 업계 동향과 세계 예측(-2035년)Exosome Therapy Market by Type of Therapeutic, Target Indication, Therapeutic Area, Route of Administration and Geography : Industry Trends and Global Forecasts, Till 2035 |
||||||
세계의 엑소좀 요법 시장 규모는 2029년 3,000만 달러에서 2040년에는 14억 달러로 성장하며, 2035년까지의 예측 기간 중 CAGR 41.1%에 달할 것으로 예측됩니다.
조직 재생 촉진, 염증 및 만성 통증 완화, 표적 특이성 등 다양한 장점으로 인해 최근 엑솜 요법이 큰 주목을 받고 있습니다. 다양한 연구를 통해 엑소좀 치료제의 질병 진단, 약물전달, 치료 적용에 있으며, 높은 효능과 치료적 우월성이 입증되고 있습니다. 현재 전 세계에서 120개 이상의 치료제 후보물질이 수많은 질병 적응증에 대한 치료를 위해 연구되고 있다는 점은 주목할 만합니다. 이는 이 분야에서 제약회사들의 광범위한 개발 노력이 이루어지고 있음을 보여줍니다. 전체의 40% 이상이 다양한 임상 개발 단계에 있습니다. 전체 후보물질 중 EXOMSC-COV19(Dermama Biotech Lab), Dex2(Gustave Roussy Institute), SF-MSC-EX(Osmangazi University)를 포함한 7개의 엑소좀 치료제가 현재 개발의 고급 임상단계에서 시험 중입니다.
현재 엑소좀을 이용한 치료제의 약 30%가 다양한 암 치료제로 개발되고 있습니다. 이 혁신적인 접근법은 엑소좀을 암세포에 특이적으로 약물을 전달하는 수단으로 사용하는 것으로, 이러한 치료법은 아직 연구 초기 단계에 있습니다. 엑소좀 전달 시스템에 대해 연구되고 있는 주요 암종으로는 유방암, 폐암, 흑색종, 대장암 등이 있습니다. 이 분야에서 진행 중인 연구개발 노력, 유망한 임상시험 결과, 후기 엑소좀 치료제의 출시 등을 고려할 때, 향후 10년간 이 시장은 괄목할 만한 성장을 이룰 것으로 예상됩니다.
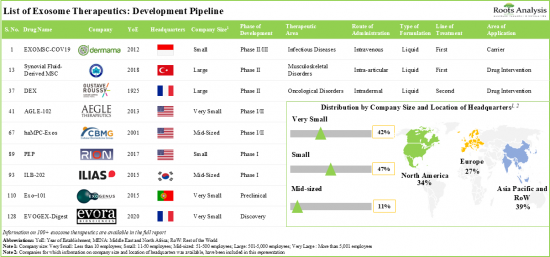
전 세계 60여개 기업이 엑소좀 치료제 개발에 착수하고 있으며, 스타트업과 소규모 기업의 존재가 시장 특징입니다.
엑소좀 관련 치료법 및 바이오마커를 평가하는 임상시험에는 다양한 지역에서 3,000명 이상의 환자가 모집/등록되어 있습니다. 현재 진행 중인 엑소좀 치료제 연구개발을 위해 530개 이상의 보조금이 지원되고 있습니다. 이 분야의 장점과 미래 가능성을 알아본 다양한 투자자들이 30개 이상의 사례에 5억 7,000만 달러 이상을 투자했습니다.
현재 이 분야는 30개 이상의 스타트업이 혁신을 주도하고 있으며, 최근 수년간 엑소좀 치료제 개발을 위해 다양한 R&D 구상이 진행되고 있습니다.
세계의 엑소좀 요법 시장에 대해 조사했으며, 시장의 개요와 치료 유형별, 적응증별, 치료 영역별, 투여 경로별, 지역별 동향 및 시장에 참여하는 기업의 개요 등을 제공하고 있습니다.
목차
제1장 서문
제2장 개요
제3장 서론
제4장 엑소좀 치료제 : 시장 구도
제5장 엑소좀 치료제 개발 기업 : 기업 개요
- Codiak BioSciences
- Coya Therapeutics
- Curexsys
- EV Therapeutics
- Evox Therapeutics
- SHIFTBIO
제6장 엑소좀 치료제 : 약제 개요
- AEGLE 세라퓨틱스
- AVEM 헬스케어
- 세포 바이오메디컬 그룹
- OBCTCD24
- 리뉴론
- 줄기세포 의료
제7장 임상시험의 분석
제8장 학술 보조금의 분석
제9장 세계 이벤트 분석
제10장 파트너십과 협업
제11장 자금조달과 투자 분석
제12장 스타트업 건강 지표
제13장 사례 연구 : 엑소좀 개발 및 제조 서비스 프로바이더
제14장 약제 실패의 분석
제15장 시장 규모의 평가와 기회 분석
제16장 이그제큐티브 인사이트
- Capricor Therapeutics
- Exogenus Therapeutics
- ILIAS Biologics
제17장 부록 1 : 표 형식 데이터
제18장 부록 2 : 기업·단체 리스트
KSA 25.02.24EXOSOME THERAPY MARKET: OVERVIEW
As per Roots Analysis, the global exosome therapy is estimated to grow from USD 0.03 billion in 2029 to USD 1.4 billion by 2040, at a CAGR of 41.1% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Exosome Therapeutics
- Allogenic
- Autologous
Target Disease Indications
- Degenerative Meniscal Injury
- Dystrophic Epidermolysis Bullosa
- Fistula Perianal
- Retinitis Pigmentosa
Therapeutic Area
- Dermatological Disorders
- Musculoskeletal Disorders
- Ophthalmic Disorders
- Rectal Disorders
Route of Administration
- Fistula Tract
- Intra-articular
- Intra-ocular
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
EXOSOME THERAPY MARKET: GROWTH AND TRENDS
Owing to the various benefits, such as enhanced tissue regeneration, reduced inflammation and chronic pain, and target specificity, exosome therapies have garnered significant attention in the recent past. Various studies have demonstrated the high efficacy and therapeutic superiority of exosome therapeutics in disease diagnosis, drug delivery and therapeutic applications. It is worth highlighting that over 120 therapeutic candidates are currently being investigated for treating myriads of disease indications, across the globe. This depicts the extensive development efforts being undertaken by drug developers in this domain. More than 40% of the total number of drugs are in various phases of clinical development. Of the total candidates, seven exosome therapies, including EXOMSC-COV19 (Dermama Biotech Lab), Dex2 (Gustave Roussy Institute), SF-MSC-EX (Osmangazi University), are currently being tested in the advanced clinical stages of development.
At present, around 30% of exosome-based therapeutics are under development for the treatment of various cancers. This innovative approach uses exosomes as a means to deliver drugs specifically to cancer cells, and these therapies are still in the early phases of research. Notable types of cancer being investigated for exosome delivery systems include breast cancer, lung cancer, melanoma, and colon cancer. Considering the ongoing R&D efforts in this domain, promising clinical trial results and anticipated launch of late-stage exosome therapies, the market is anticipated to witness notable growth over the next decade.
EXOSOME THERAPY MARKET: KEY INSIGHTS
The report delves into the current state of the exosome therapy market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Close to 60 players, worldwide, have taken initiatives to develop exosome therapeutics; the market is characterized by the presence of start-ups and small companies.
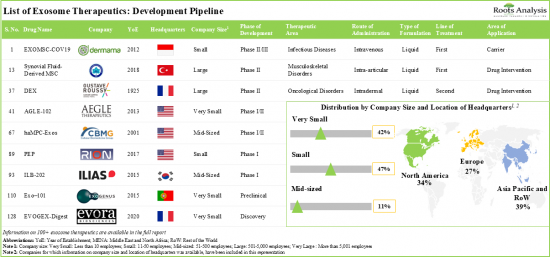
- The current pipeline features more than 120 exosome therapeutics that are being evaluated across different phases of development; most of these are designed for intravenous administration.
- 3,000+ patients have been recruited / enrolled in clinical trials evaluating exosome related therapies and biomarkers across different geographies.
- More than 530 grants have been awarded for the ongoing R&D efforts for exosome therapeutics; the University of California has been awarded the maximum grant amount of USD 21 million.
- A variety of investors, having realized the benefits and future opportunities in this field, have invested more than USD 570 million across more than 30 instances.
- The rising interest of stakeholders in exosome therapeutics is also reflected by the increasing number of partnerships established by various industry and non-industry players.
- Stakeholders have participated in various global events to discuss the research outcomes, and affiliated challenges as well as opportunities existing in this domain.
- At present, more than 30 start-ups are driving innovation in this domain; a variety of R&D initiatives have been undertaken by these players over the last few years for the development of exosome therapeutics.
- Lack of efficacy, the COVID-19 pandemic, limited patient enrollment, and scarce funding are among the key reasons that have led to the discontinuation of studies sponsored by various industry and non-industry players.
- With the rising demand for therapeutic advances in drug safety, the market for exosome therapeutics is expected to grow at an annualized rate of 41.1% between 2029-2040.
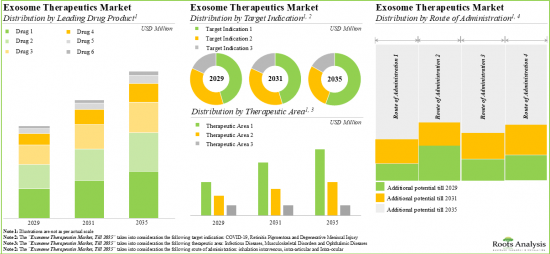
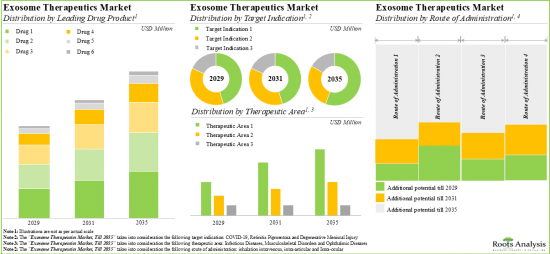
EXOSOME THERAPY MARKET: KEY SEGMENTS
Autologous Therapies are Likely to Capture Largest Share of the Exosome Therapy Market
Based on the type of therapy, the market is segmented into allogenic therapy and autologous therapy. It is anticipated that the autologous therapy segment will hold the maximum share of the exosome therapy market in 2029. However, it is worth highlighting that allogeneic therapy is likely to grow at a relatively higher CAGR.
Retinitis Pigmentosa is Likely to be the Fastest Growing Segment of the Exosome Therapy Market During the Forecast Period
Based on the target disease indication, the market is segmented into degenerative meniscal injury, dystrophic epidermolysis bullosa, fistula perianal and retinitis pigmentosa. It is anticipated that fistula perianal will hold the maximum share of the exosome therapy market in 2029. However, it is worth highlighting that the exosome therapy market for retinitis pigmentosa is likely to grow at a relatively higher CAGR.
Rectal Disorders Segment is the Fastest Growing Segment of the Exosome Therapy Market
Based on the therapeutic area, the market is segmented into dermatological disorders, musculoskeletal disorders, ophthalmic disorders and rectal disorders. While the rectal disorders segment is likely to hold a relatively higher market share, it is worth highlighting that the ophthalmic diseases segment is expected to witness substantial market growth in the coming years.
Fistula Tract Segment is Likely to Capture Largest Share of the Exosome Therapy Market in 2029
Based on the route of administration, the market is segmented into fistula tract, intra-articular and intra-ocular. It is anticipated that the fistula tract segment will hold the maximum share of the exosome therapy market in 2029. However, it is worth highlighting that the intraocular segment is likely to grow at a relatively higher CAGR.
Asia-Pacific Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and the Rest of the World. The majority share is expected to be captured by drug developers based in Asia-Pacific. It is worth highlighting that, over the years, the market in Europe is expected to grow at a higher CAGR.
Example Players in the Exosome Therapy Market
- Coya Therapeutics
- Evox Therapeutics
- Curexsys
- EV Therapeutics
- SHIFTBIO
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Chief Business Officer, Capricor Therapeutics
- R&D and Innovation Manager, Exogenus Therapeutics
- Chief Business Officer, ILIAS Biologics
EXOSOME THERAPY MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the exosome therapy market, focusing on key market segments, including [A] type of therapy, [B] target disease indications, [C] therapeutic area, [D] route of administration and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of exosome therapeutics, considering various parameters, such as [A] phase of development, [B] technology platform, [C] type of payload, [D] target disease indication (s), [E] therapeutic area, [F] biological target, [G] route of administration, [H] type of therapy, [I] combination drug, [J] line of treatment and [K] dosing frequency. Further, it includes details on the exosome therapeutic developers, along with information on their [L] year of establishment, [M] company size and [N] location of headquarters.
- Company Profiles: In-depth profiles of key industry players engaged in the development of exosome therapeutics, focusing on [A] company overviews, [B] financial information (if available), [C] product portfolio, [E] recent developments and [F] an informed future outlook.
- Drug Profiles: In-depth profiles of key exosome therapies, focusing on [A] product portfolio and [B] clinical trial information.
- Clinical Trials Analysis: Examination of completed, ongoing, and planned clinical studies of various exosome therapeutics, based on parameters like [A] trial status, [B] trial registration year, [C] type of sponsor / collaborator, [D] study design, [E] number of patients enrolled, [F] year-wise trend of completed and recruiting trials, [G] age group of the patients enrolled, [H] active industry and non-industry players and [I] location of the trials.
- Academic Grant Analysis: A comprehensive examination of various academic grants that have been awarded to various research institutes for projects related to exosome therapeutics, based on several parameters, such as [A] year of grant awarded, [B] amount awarded, [C] type of funding institute center, [D] popular NIH departments, [E] support period, [F] emerging focus area, [G] purpose of grants, [H] grant activity code, [I] local recipients, [J] type of recipient organization study section, [K] type of grant application and [L] popular recipient organizations, (in terms of number of grants and amount awarded).
- Global Event Analysis: An in-depth analysis of the global events attended by the exosome therapy developers, based on several relevant parameters, such as [A] year of event, [B] type of event platform, [C] location of event, [D] emerging focus areas, [E] active organizers (in terms of number of events), [F] active industry and non-industry participants, [G] designation of participants, [H] affiliated department of participants, and [I] active speakers (in terms of number of events).
- Partnerships and Collaborations: An analysis of partnerships established in this sector, since 2017, covering research agreements, licensing agreements, manufacturing agreements, product development and commercialization agreements, mergers / acquisitions, and other relevant deals.
- Funding and Investment Analysis: A detailed evaluation of the investments made in this domain, encompassing seed financing, venture capital financing, IPOs, secondary offering, debt financing and other equity.
- Start-Up Health Indexing: An analysis of start-ups / small companies engaged in the development of exosome therapeutics, based on parameters, such as [A] pipeline strength, [B] pipeline maturity, [C] financial support, [D] number of investors, [E] partnership activity and [F] start-up health indexing.
- Case Study: A case study highlighting the companies offering development and manufacturing services for exosome therapeutics, along with the information on their [A] year of establishment, [B] company size, [C] location of headquarters, [D] types of service(s) offered, [E] method of isolation, [F] method of purification, [G] method of characterization, [H] method of exosome manufacturing, [I] scale of operation and [J] scalability.
- Drug Failure Analysis: An in-depth analysis, focusing on exosome therapeutics that failed to progress to later stages of clinical development, based on various relevant parameters, such as [A] trial status of discontinuation, [B] target disease indication(s), [C] route of administration and [D] type of sponsor.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Key Questions Answered
- 1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Overview of Exosomes
- 3.1.1. Types of Extracellular Vesicles
- 3.1.2. Potential Sources of Exosomes
- 3.2. Exosome Biogenesis
- 3.2.1. Exosome Formation and Development Process
- 3.2.2. Secretion of Exosomes
- 3.3. Applications of Exosomes
- 3.4. Mechanism of Exosome Therapy
- 3.4.1. Exosome Drug Therapy
- 3.4.2. Exosome RNAi Therapy
- 3.4.3. Exosome Immunotherapy
- 3.5. Advantages of Exosome Therapies
- 3.6. Risks and Future Perspectives Associated with Exosome Therapeutics
4. EXOSOME THERAPEUTICS: MARKET LANDSCAPE
- 4.1. Analysis Methodology and Key Parameters
- 4.2. Exosome Therapeutics Market Landscape
- 4.2.1. Analysis by Phase of Development
- 4.2.2. Analysis by Technology Platform
- 4.2.3. Analysis by Type of Payload
- 4.2.4. Analysis by Type of Formulation
- 4.2.5. Analysis by Type of Therapy
- 4.2.6. Analysis by Derived Source
- 4.2.7. Analysis by Target Disease Indication(s)
- 4.2.8. Analysis by Therapeutic Area
- 4.2.9. Analysis by Phase of Development and Therapeutic Area
- 4.2.10. Analysis by Route of Administration
- 4.2.11. Analysis by Therapeutic Area and Route of Administration
- 4.2.12. Analysis by Type of Therapy (By Method of Composition)
- 4.2.13. Analysis by Line of Treatment
- 4.2.14. Analysis by Dosing Frequency
- 4.3. Exosome Therapeutics Developers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters
- 4.3.4. Most Active Players: Analysis by Number of Therapeutics
5. EXOSOME THERAPEUTICS DEVELOPERS: COMPANY PROFILES
- 5.1. Codiak BioSciences
- 5.1.1. Company Overview
- 5.1.2. Product Portfolio
- 5.1.3. Clinical Trial Information
- 5.1.4. Recent Developments and Future Outlook
- 5.2. Coya Therapeutics
- 5.2.1. Company Overview
- 5.2.2. Product Portfolio
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Curexsys
- 5.3.1. Company Overview
- 5.3.2. Product Portfolio
- 5.3.3. Recent Developments and Future Outlook
- 5.4. EV Therapeutics
- 5.4.1. Company Overview
- 5.4.2. Product Portfolio
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Evox Therapeutics
- 5.5.1. Company Overview
- 5.5.2. Product Portfolio
- 5.5.3. Recent Developments and Future Outlook
- 5.6. SHIFTBIO
- 5.6.1. Company Overview
- 5.6.2. Product Portfolio
- 5.6.3. Recent Developments and Future Outlook
6. EXOSOME THERAPEUTICS: DRUG PROFILES
- 6.1. AEGLE Therapeutics
- 6.1.1. Company Overview
- 6.1.2. AGLE-102: Product Portfolio
- 6.1.2.1. AGLE-102: Clinical Trial Information
- 6.1.3. Recent Developments and Future Outlook
- 6.2. AVEM Healthcare
- 6.2.1. Company Overview
- 6.2.2. Ardoxso: Product Portfolio
- 6.2.2.1. Ardoxso: Clinical Trial Information
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Cellular Biomedicine Group
- 6.3.1. Company Overview
- 6.3.2. Financial Information
- 6.3.3. haMPC-Exos: Product Portfolio
- 6.3.3.1. haMPC-Exos: Clinical Trial Information
- 6.3.4. hMSC-Exos: Product Portfolio
- 6.3.4.1. hMSC-Exos: Clinical Trial Information
- 6.3.5. Undisclosed Drug 1: Product Portfolio
- 6.3.5.1. Undisclosed Drug 1: Clinical Trial Information
- 6.3.6. Recent Developments and Future Outlook
- 6.4. OBCTCD24
- 6.4.1. Company Overview
- 6.4.2. CovenD24: Product Portfolio
- 6.4.2.1. CovenD24: Clinical Trial Information
- 6.4.3. Recent Developments and Future Outlook
- 6.5. ReNeuron
- 6.5.1. Company Overview
- 6.5.2. Financial Information
- 6.5.3. Undisclosed Drug 1: Product Portfolio
- 6.5.3.1. Undisclosed Drug 1: Clinical Trial Information
- 6.5.4. Recent Developments and Future Outlook
- 6.6. Stem Cell Medicine
- 6.6.1. Company Overview
- 6.6.2. Undisclosed Drug 1: Product Portfolio
- 6.6.3. Recent Developments and Future Outlook
7. CLINICAL TRIAL ANALYSIS
- 7.1. Analysis Methodology and Key Parameters
- 7.2. Exosome Therapeutics: List of Clinical Trials
- 7.2.1. Analysis by Trial Status
- 7.2.2. Analysis by Trial Registration Year
- 7.2.3. Analysis by Type of Sponsor / Collaborator
- 7.2.4. Analysis by Registration Year and Type of Study
- 7.2.5. Analysis by Registration Year and Status of Trial
- 7.2.6. Analysis by Study Design
- 7.2.7. Analysis by Patient Enrollment
- 7.2.8. Year-wise Trend of Completed and Recruiting Trials
- 7.2.9. Analysis by Age Category
- 7.2.10. Analysis by Phase of Development and Trial Status
- 7.2.11. Analysis by Phase of Development and Patient Enrollment
- 7.2.12. Most Active Industry Players: Analysis by Number of Registered Trials
- 7.2.13. Most Active Non-Industry Players: Analysis by Number of Registered Trials
- 7.2.14. Analysis by Trial Location
- 7.2.15. Analysis by Trial Status and Geography
8. ACADEMIC GRANT ANALYSIS
- 8.1. Analysis Methodology and Key Parameters
- 8.2. Exosome Therapeutics: List of Academic Grants
- 8.3. Analysis by Year of Grants Awarded
- 8.4. Analysis by Amount Awarded
- 8.5. Popular NIH Departments: Analysis by Number of Grants
- 8.6. Analysis by Type of Funding Institute Center
- 8.7. Analysis by Support Period
- 8.8. Analysis by Purpose of Grants
- 8.9. Word Cloud Analysis: Emerging Focus Area
- 8.10. Analysis by Grant Activity Code
- 8.11. Analysis by Local Recipients
- 8.12. Analysis by Type of Recipient Organization
- 8.13. Popular Recipient Organizations: Analysis by Amount Awarded
- 8.14. Popular Recipient Organizations: Analysis by Number of Grants
- 8.15. Analysis by Study Section Involved
- 8.16. Analysis by Type of Grant Application
- 8.17. Analysis by Funding Institute Center and Support Year
9. GLOBAL EVENT ANALYSIS
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.3. List of Global Events Related to Exosomes
- 9.3.1. Analysis by Year of Event
- 9.3.2. Analysis by Event Platform
- 9.3.3. Analysis by Type of Event
- 9.3.4. Analysis by Location of Event
- 9.3.5. Word Cloud Analysis: Evolutionary Trends in Event Agenda / Key Focus Area
- 9.3.6. Most Active Organizers: Analysis by Number of Events
- 9.3.7. Most Active Industry Participants: Analysis by Number of Events
- 9.3.8. Most Active Non-Industry Participants: Analysis by Number of Events
- 9.3.9. Analysis by Designation of Participant
- 9.3.10. Analysis by Affiliated Department of Participant
- 9.3.11. Most Active Speakers: Analysis by Number of Events
10. PARTNERSHIPS AND COLLABORATIONS
- 10.1. Chapter Overview
- 10.2. Partnership Models
- 10.3. Exosome Therapeutics: List of Partnerships and Collaborations
- 10.3.1. Analysis by Year of Partnership
- 10.3.2. Analysis by Type of Partnership
- 10.3.3. Analysis by Year and Type of Partnership
- 10.3.4. Analysis by Company and Type of Partnership
- 10.3.5. Analysis by Type of Technology Platform
- 10.3.6. Analysis by Type of Partner
- 10.3.7. Word Cloud Analysis: Emerging Focus Areas
- 10.3.8. Analysis by Target Disease Indication(s)
- 10.3.9. Analysis by Therapeutic Area
- 10.3.10. Analysis by Therapeutic Area and Type of Partnership
- 10.3.11. Most Active Players: Analysis by Number of Partnerships
- 10.3.12. Regional Analysis
- 10.3.13. Intercontinental and Intracontinental Agreements
11. FUNDING AND INVESTMENT ANALYSIS
- 11.1. Analysis Methodology and Key Parameters
- 11.2. Type of Funding
- 11.3. Exosome Therapeutics: List of Funding Instances
- 11.4. Analysis by Year of Funding
- 11.5. Analysis by Amount Invested
- 11.6. Analysis by Type of Funding
- 11.7. Analysis by Type of Funding and Amount Invested
- 11.8. Analysis by Year, Type of Funding and Amount Invested
- 11.9. Analysis by Purpose of Funding
- 11.10. Analysis by Target Disease Indication(s)
- 11.11. Analysis by Therapeutic Area
- 11.12. Most Active Players: Analysis by Number of Funding Instances
- 11.13. Most Active Players: Analysis by Amount Invested
- 11.14. Key Investors: Analysis by Number of Funding Instances
- 11.15. Regional Analysis
- 11.16. Summary of Funding Instances
12. START-UP HEALTH INDEXING
- 12.1. Analysis Methodology and Key Parameters
- 12.2. Analysis by Pipeline Maturity
- 12.3. Analysis by Pipeline Strength
- 12.4. Analysis by Financial Support
- 12.5. Analysis by Number of Investors
- 12.6. Analysis by Partnership Activity
- 12.7. Start-up Health Indexing: Roots Analysis Perspective
13. CASE STUDY: EXOSOME DEVELOPMENT AND MANUFACTURING SERVICE PROVIDERS
- 13.1. Chapter Overview
- 13.2. Exosome Development and Manufacturing Service Providers Landscape
- 13.2.1. Analysis by Year of Establishment
- 13.2.2. Analysis by Company Size
- 13.2.3. Analysis by Location of Headquarters
- 13.2.4. Analysis by Location of Headquarters and Company Size
- 13.3. Analysis by Type of Service(s) Offered
- 13.3.1. Analysis by Method of Isolation
- 13.3.2. Analysis by Method of Purification
- 13.3.3. Analysis by Method of Characterization
- 13.3.4. Analysis by Method of Exosome Manufacturing
- 13.3.5. Analysis by Scale of Operation
- 13.3.6. Analysis by Scalability
14. DRUG FAILURE ANALYSIS
- 14.1. Methodology and Key Parameters
- 14.2. Exosome Therapeutics: List of Failed Drug Candidates
- 14.2.1. Analysis by Study Start Year and Year of Termination of the Clinical Trials
- 14.2.2. Analysis by Trial Status of Discontinuation
- 14.2.3. Analysis by Target Disease Indication(s)
- 14.2.4. Analysis by Route of Administration
- 14.2.5. Analysis by Type of Sponsor
- 14.2.6. Analysis by Reasons for Drug Failure
- 14.2.7. Word Cloud Analysis: Emerging Focus Area
15. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 15.1. Forecast Methodology and Key Assumptions
- 15.2. Global Exosome Therapeutics Market, 2029-2040
- 15.3. Global Exosome Therapeutics Market, 2029-2040: Distribution by Target Disease Indication(s)
- 15.3.1. Exosome Therapeutics Market for COVID-19, 2029-2040
- 15.3.2. Exosome Therapeutics Market for Degenerative Meniscal Injury, 2029-2040
- 15.3.3. Exosome Therapeutics Market for Retinitis Pigmentosa, 2029-2040
- 15.4. Global Exosome Therapeutics Market, 2029-2040: Distribution by Therapeutic Area
- 15.4.1. Exosome Therapeutics Market for Infectious Diseases, 2029-2040
- 15.4.2. Exosome Therapeutics Market for Ophthalmic Diseases, 2029-2040
- 15.4.3. Exosome Therapeutics Market for Musculoskeletal Disorders, 2029-2040
- 15.5. Global Exosome Therapeutics Market, 2029-2040: Distribution by Route of Administration
- 15.5.1. Intravenous Exosome Therapeutics Market, 2029-2040
- 15.5.2. Intra-ocular Exosome Therapeutics Market, 2029-2040
- 15.5.3. Intra-articular Exosome Therapeutics Market, 2029-2040
- 15.5.4. Inhalation Exosome Therapeutics Market, 2029-2040
- 15.6. Global Exosome Therapeutics Market, 2029-2040: Distribution by Type of Formulation
- 15.6.1. Exosome Therapeutics Market for Liquid Formulations, 2029-2040
- 15.6.2. Exosome Therapeutics Market for Aerosol Formulations, 2029-2040
- 15.7. Global Exosome Therapeutics Market, 2029-2040: Distribution by Geography
- 15.7.1. Exosome Therapeutics Market in North America, 2029-2040
- 15.7.2. Exosome Therapeutics Market in Europe, 2029-2040
- 15.7.3. Exosome Therapeutics Market in Asia Pacific, 2030-2040
- 15.8. Attractiveness and Competitiveness Matrix
16. EXECUTIVE INSIGHTS
- 16.1. Capricor Therapeutics
- 16.1.1. Company Snapshot
- 16.1.2. Interview Transcript: Chief Business Officer
- 16.2. Exogenus Therapeutics
- 16.2.1. Company Snapshot
- 16.2.2. Interview Transcript: R&D and Innovation Manager
- 16.3. ILIAS Biologics
- 16.3.1. Company Snapshot
- 16.3.2. Interview Transcript: Chief Business Officer



















