
|
시장보고서
상품코드
1585174
바이오시밀러 시장 : 약효군별, 치료영역별, 제조업체 유형별, 유통채널별, 지역별, 주요 기업별 - 업계 동향 및 세계 예측(-2035년)Global Biosimilars Market - Distribution by Drug Class, Therapeutic Area, Type of Manufacturer, Distribution Channel, Geographical Regions and Leading Players: Industry Trends and Global Forecasts, till 2035 |
||||||
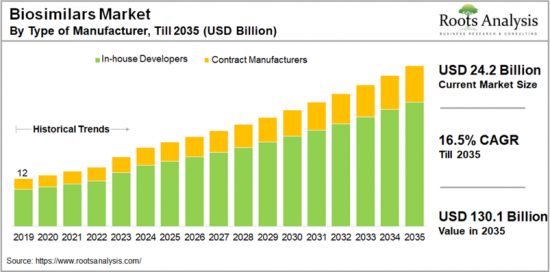
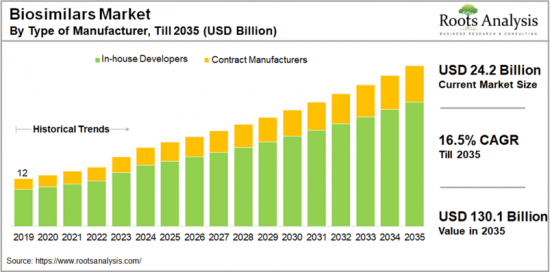
세계 바이오시밀러 시장 규모는 2024년 24억 2,000만 달러에 달할 것으로 예상되며, 2035년까지의 예측 기간 중 16.5%의 연평균 복합 성장률(CAGR)로 확대될 것으로 예상됩니다.
최근 생물제제 시장은 만성질환 관리의 효능 향상으로 인해 크게 성장하고 있습니다. 그러나 생물학적 제제와 관련된 높은 비용은 경제와 의료에 큰 도전이 되고 있습니다. 생물학적 제제에 대한 수요가 계속 증가함에 따라 개발사들은 투자수익률을 높이기 위한 혁신적인 전략을 모색하는 한편, 유사한 안전성과 효능을 가진 보다 저렴한 가격의 대체 생물학적 제제에 대한 필요성에 대해 고민하고 있습니다. 특허 만료, 의약품 파이프라인 축소, 치료비 절감의 압박 속에서 바이오시밀러 의약품은 바이오의약품 산업에서 큰 비중을 차지할 것으로 예상됩니다. 이러한 추세는 지난 수년간 바이오시밀러 개발 기업 간의 투자와 제휴가 급증한 것도 지원하고 있습니다.
또한 승인 절차의 간소화와 바이오시밀러 개발을 위한 명확한 가이드라인의 확립과 같은 규제 발전은 바이오시밀러 개발을 촉진하고 있습니다. 또한 바이오시밀러와 유사한 안전성과 효능을 가진 참조 생물학적 제제와의 대체 가능성을 평가하는 호환성 연구도 바이오시밀러 개발을 촉진하는 데 중요한 역할을 하고 있습니다. 또한 비용 효율적인 대체품으로 바이오시밀러에 대한 수요가 증가함에 따라 자체 개발 및 아웃소싱의 필요성이 증가하고 있으며, 이는 바이오시밀러 개발 기업에게 큰 성장 기회로 작용하고 있습니다.
세계의 바이오시밀러 시장에 대해 조사했으며, 시장의 개요와 약제 클래스별, 치료 영역별, 제조업체 유형별, 유통 채널별, 지역별 동향 및 시장에 참여하는 기업의 개요 등을 제공하고 있습니다.
목차
제1장 서문
제2장 조사 방법
제3장 시장 역학
제4장 경제 및 기타 프로젝트 특유 고려 사항
제5장 개요
제6장 서론
제7장 시장 구도
- 장의 개요
- 바이오시밀러 : 개발업자의 상황
- 바이오시밀러 : 시장 구도
제8장 기업 개요 : 북미에 기반한 바이오시밀러 개발 기업
- 장의 개요
- Amgen
- Coherus BioSciences
- Eli Lilly
- Pfizer
제9장 기업 개요 : 유럽에 기반한 바이오시밀러 개발 기업
- 장의 개요
- BIOCAD
- Fresenius Kabi
- Sandoz
- STADA
제10장 기업 개요 : 아시아태평양 및 기타 지역에 기반한 바이오시밀러 개발 기업
- 장의 개요
- Biocon
- Dr. Reddy's Laboratories
- Celltrion
- Intas Pharmaceuticals
- Teva Pharmaceuticals
제11장 규제 상황
- 장의 개요
- 북미에서 바이오시밀러의 규제 가이드라인
- 유럽에서 바이오시밀러의 규제 가이드라인
- 아시아태평양에서 바이오시밀러의 규제 가이드라인
- 향후 전망
제12장 원가분석
- 장의 개요
- 신규 생물제제의 고가격화에 기여하는 요인
- 바이오시밀러의 가격 설정
- 결론
제13장 사례 연구 : 바이오시밀러의 아웃소싱
제14장 시장 영향 분석
제15장 세계의 바이오시밀러 시장
제16장 바이오시밀러 시장(약제 클래스별)
제17장 바이오시밀러 시장(치료 영역별)
제18장 바이오시밀러 시장(제조업체 유형별)
제19장 바이오시밀러 시장(유통 채널별)
제20장 바이오시밀러 시장(지역별)
제21장 바이오시밀러 시장(주요 기업별)
제22장 결론
제23장 부록 I : 표 형식 데이터
제24장 부록 II : 기업 및 단체 리스트
KSA 24.11.19The global biosimilars market is valued at USD 24.2 billion in 2024, growing at a CAGR of 16.5% during the forecast period, till 2035.
In recent years, the biologics market has experienced significant growth, owing to its improved effectiveness in managing chronic diseases. However, the high costs associated with biologics pose considerable economic and healthcare challenges. As the demand for biologics continues to increase, developers are exploring innovative strategies to enhance returns on investment while addressing the need for more affordable alternative biological products that encompass similar safety and efficacy profiles. Amidst patent expirations, shrinking drug pipelines, and pressure to reduce the treatment costs, biosimilars are expected to occupy a significant share within the biopharmaceutical industry. This trend is further supported by surge in investments and collaborations among biosimilar developers in the past few years. Further, regulatory advancements, such as streamlining approval processes and establishing clearer guidelines for biosimilar development have facilitated the development of biosimilars. Moreover, studies on interchangeability, which assess the feasibility of substituting biosimilars with reference biologics with similar safety or efficacy, have also played a critical role in advancing biosimilar development. In addition, the rising demand for biosimilars as a cost-effective alternative has promoted the need for increased in-house development and outsourcing operations, thereby creating significant growth opportunities for biosimilar developers.
Key Market Segments
Drug Class
- Monoclonal Antibodies
- Proteins
- Peptides
- Others
Therapeutic Area
- Oncological Disorders
- Autoimmune and Inflammatory Disorders
- Hematological Disorders
- Metabolic Disorders
- Other Disorders
Type of Manufacturer
- Contract Manufacturers
- In-house Developers
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Geographical Regions
- North America (US and Canada)
- Europe (Germany, UK, France, Spain, Italy, Switzerland, Belgium, Denmark and Rest of Europe)
- Asia-Pacific (China, India, Japan, South Korea and Australia)
- Middle East and North Africa (Saudi Arabia, Egypt and UAE)
- Latin America (Brazil, Mexico and Argentina)
Research Coverage:
- A preface providing an overview of the report, Global Biosimilars Market (2nd Edition), till 2035.
- An overview of the systematic research methodology adopted in this study, which includes research assumptions, forecasting methodologies, primary and secondary research techniques, as well as the various analytical frameworks integrated into the report.
- A summary of the comprehensive methodologies and frameworks used to forecast and analyze market trends, examining key factors that influence market dynamics while emphasizing the rigorous quality control measures implemented to ensure transparency and credibility in the insights presented throughout the report.
- A brief overview of the economic factors affecting the biosimilars market, including currency fluctuations, foreign exchange rates, and existing trade barriers. Additionally, it assesses the impact of global recession and inflation on market growth, drawing insights from significant historical events to inform future decision-making.
- An executive summary of the insights obtained during our research, featuring key takeaways from the current state of the biosimilars industry and its likely evolution in the short to mid-to-long term.
- A general overview of biosimilars, highlighting the key differences between innovator biologics, biosimilars, and generics. Further, it also provides information on the need for biosimilars, the manufacturing process, development timelines, and future perspectives related to the evolution of the biosimilar industry.
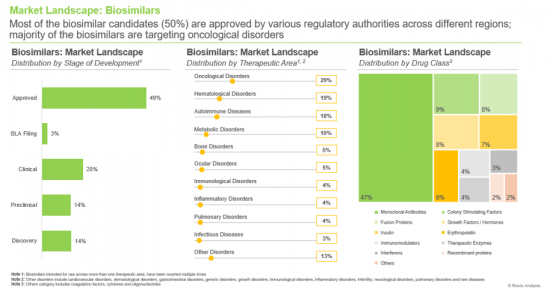
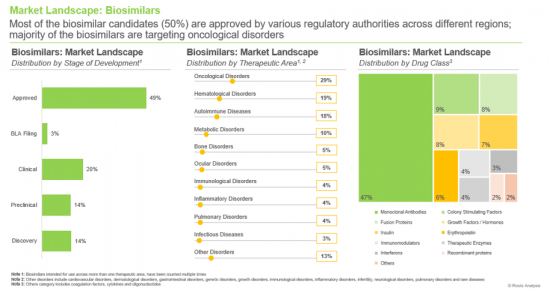
- A comprehensive evaluation of marketed and clinical-stage biosimilars based on several parameters, including the stage of development (approved, BLA registration, clinical, preclinical and discovery), therapeutic area (autoimmune diseases, bone disorders, cardiovascular disorders, growth disorders, hematological disorders, immunological disorders, infectious diseases, infertility, inflammatory disorders, metabolic disorders, neurological disorders, ocular disorders, oncological disorders, pulmonary disorders, rare diseases and other disorders), drug class (coagulation factors, colony stimulating factors, cytokines, erythropoietin, fusion proteins, growth hormones / factors, immunomodulators, insulin, interferons, monoclonal antibodies, oligonucleotides, recombinant proteins, therapeutic enzymes, vaccines and unspecified). Additionally, it includes detailed analyses of biosimilar developers based on parameters, such as year of establishment, company size, headquarters location, and the most active players (in terms of number of biosimilars developed).
- Detailed profiles of prominent companies in North America, Europe, Asia-Pacific, and the rest of the world. Each profile includes an overview of the company (year of establishment, headquarters location, employee count, leadership team, business segments), financial information (if available), biosimilar pipeline details, recent developments and future outlook.
- A brief overview of the regulatory guidelines established by regulatory bodies across different regions, such as North America, Europe, and Asia-Pacific. It also presents details on regulatory approval pathways issued by authorities in these regions.
- An analysis of the factors contributing to the high pricing of novel biologics, including factors influencing biosimilar pricing. This section also presents a price comparison between various biosimilars and their reference biologics.
- A case study focused on the growing global biosimilars market and associated opportunities for biologics CMOs and CDMOs. It discusses the need for outsourcing manufacturing operations for biosimilar drugs and examines the impact of biosimilars on the global contract manufacturing market, along with the associated challenges and future perspectives.
- An in-depth analysis of factors impacting the growth of the biosimilars industry. This includes information on key drivers, potential restraints, emerging opportunities, and existing challenges within the sector.
- A detailed estimation of the current market size and future growth potential within the biosimilars market over the next decade. Based on various parameters and validations from reliable secondary and primary sources, we've provided informed estimates on market evolution till 2035. The report also features the likely distribution of the current and forecasted opportunities along with three forecast scenarios, namely conservative, base, and optimistic, representing different growth trajectories.
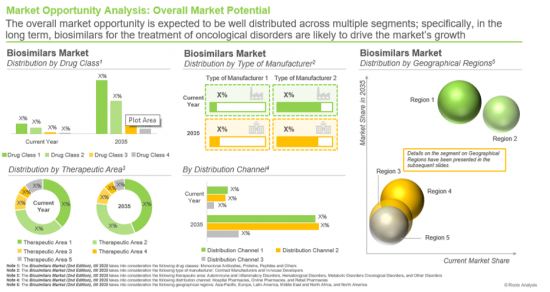
- A detailed estimation of the current market size and future opportunities in the biosimilars market across various drug classes, such as monoclonal antibodies, proteins, peptides, and others.
- A detailed projection of current market size and future opportunities in the biosimilar market across different therapeutic areas, including oncological disorders, autoimmune and inflammatory disorders, hematological disorders, metabolic disorders, and others.
- A detailed projection of current market size and future opportunities in the biosimilars market across different types of manufacturers, such as contract manufacturers and in-house developers.
- A detailed projection of the current market size and future opportunities in the biosimilars market across different distribution channels, such as hospital pharmacies, retail pharmacies and online pharmacies.
- A detailed projection of current market size and future opportunities in the biosimilars market across different geographical regions such as North America, Europe, Asia-Pacific, Middle East and North Africa (MENA), and Latin America.
- Detailed information on leading players engaged in the development of biosimilars.
Key Benefits of Buying this Report
- The report offers valuable insights into revenue estimation for both the overall market and its sub-segments, in order to empower market leaders and newcomers with critical information requisite for establishing their footprint in the industry.
- Stakeholders can utilize the report to enhance their understanding of the competitive landscape, allowing for improved business positioning and more effective go-to-market strategies.
- The report provides stakeholders with an overview of the global biosimilars market, furnishing them with essential information on significant market drivers, barriers, opportunities, and challenges.
Example Companies Profiled
- Amgen Inc
BIOCAD
- Biocon
- Celltrion
- Coherus BioSciences
- Dr. Reddy's Laboratories
- Eli Lilly
- Fresenius Kabi
- Intas Pharmaceuticals
- Pfizer Inc
- Sandoz
STADA
- Teva Pharmaceuticals
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Forecast Methodology
- 3.2.1. Top-down Approach
- 3.2.2. Bottom-up Approach
- 3.2.3. Hybrid Approach
- 3.3. Market Assessment Framework
- 3.3.1. Total Addressable Market (TAM)
- 3.3.2. Serviceable Addressable Market (SAM)
- 3.3.3. Serviceable Obtainable Market (SOM)
- 3.3.4. Currently Acquired Market (CAM)
- 3.4. Forecasting Tools and Techniques
- 3.4.1. Qualitative Forecasting
- 3.4.2. Correlation
- 3.4.3. Regression
- 3.4.4. Extrapolation
- 3.4.5. Convergence
- 3.4.6. Sensitivity Analysis
- 3.4.7. Scenario Planning
- 3.4.8. Data Visualization
- 3.4.9. Time Series Analysis
- 3.4.10. Forecast Error Analysis
- 3.5. Key Considerations
- 3.5.1. Demographics
- 3.5.2. Government Regulations
- 3.5.3. Reimbursement Scenarios
- 3.5.4. Market Access
- 3.5.5. Supply Chain
- 3.5.6. Industry Consolidation
- 3.5.7. Pandemic / Unforeseen Disruptions Impact
- 3.6. Key Market Segmentation
- 3.7. Robust Quality Control
- 3.8. Limitations
4. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Future Estimates
- 4.2.2. Currency Coverage and Foreign Exchange Rate
- 4.2.2.1. Major Currencies Affecting the Market
- 4.2.2.2. Factors Affecting Currency Fluctuations and Foreign Exchange Rates
- 4.2.2.3. Impact of Foreign Exchange Rate Volatility on the Market
- 4.2.2.4. Strategies for Mitigating Foreign Exchange Risk
- 4.2.3. Trade Policies
- 4.2.3.1. Impact of Trade Barriers on the Market
- 4.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 4.2.4. Recession
- 4.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.1. Time Period
5. EXECUTIVE SUMMARY
6. INTRODUCTION
- 6.1. Chapter Overview
- 6.2. Overview of Biologics
- 6.3. Overview of Biosimilars and Biobetters
- 6.4. Difference between Innovator Biologics, Biosimilars and Generics
- 6.5. Advantages of Biosimilars
- 6.6. Manufacturing of Biosimilars
- 6.7. Development Timeline of Biosimilars
- 6.8. Future Perspectives
7. MARKET LANDSCAPE
- 7.1. Chapter Overview
- 7.2. Biosimilars: Developers Landscape
- 7.2.1. Analysis by Year of Establishment
- 7.2.2. Analysis by Company Size
- 7.2.3. Analysis by Location of Headquarters
- 7.3. Biosimilars: Overall Market Landscape
- 7.3.1. Analysis by Stage of Development
- 7.3.2. Analysis by Therapeutic Area
- 7.3.3. Analysis by Drug Class
8. COMPANY PROFILES: BIOSIMILAR DEVELOPERS BASED IN NORTH AMERICA
- 8.1. Chapter Overview
- 8.2. Amgen
- 8.2.1. Company Overview
- 8.2.2. Financial Information
- 8.2.3. Biosimilar Pipeline
- 8.2.4. Recent Developments and Future Outlook
- 8.3. Coherus BioSciences
- 8.3.1. Company Overview
- 8.3.2. Financial Information
- 8.3.3. Biosimilar Pipeline
- 8.3.4. Recent Developments and Future Outlook
- 8.4. Eli Lilly
- 8.4.1. Company Overview
- 8.4.2. Financial Information
- 8.4.3. Biosimilar Pipeline
- 8.4.4. Recent Developments and Future Outlook
- 8.5. Pfizer
- 8.5.1. Company Overview
- 8.5.2. Financial Information
- 8.5.3. Biosimilar Pipeline
- 8.5.4. Recent Developments and Future Outlook
9. COMPANY PROFILES: BIOSIMILAR DEVELOPERS BASED IN EUROPE
- 9.1 Chapter Overview
- 9.2. BIOCAD
- 9.2.1. Company Overview
- 9.2.2. Biosimilar Pipeline
- 9.2.3. Recent Developments and Future Outlook
- 9.3. Fresenius Kabi
- 9.3.1. Company Overview
- 9.3.2. Financial Information
- 9.3.3. Biosimilar Pipeline
- 9.3.4. Recent Developments and Future Outlook
- 9.4. Sandoz
- 9.4.1. Company Overview
- 9.4.2. Financial Information
- 9.4.3. Biosimilar Pipeline
- 9.4.4. Recent Developments and Future Outlook
- 9.5. STADA
- 9.5.1. Company Overview
- 9.5.2. Financial Information
- 9.5.3. Biosimilar Pipeline
- 9.5.4. Recent Developments and Future Outlook
10. COMPANY PROFILES: BIOSIMILAR DEVELOPERS BASED IN ASIA-PACIFIC AND REST OF THE WORLD
- 10.1 Chapter Overview
- 10.2. Biocon
- 10.2.1. Company Overview
- 10.2.2. Financial Information
- 10.2.3. Biosimilar Pipeline
- 10.2.4. Recent Developments and Future Outlook
- 10.3. Dr. Reddy's Laboratories
- 10.3.1. Company Overview
- 10.3.2. Financial Information
- 10.3.3. Biosimilar Pipeline
- 10.3.4. Recent Developments and Future Outlook
- 10.4. Celltrion
- 10.4.1. Company Overview
- 10.4.2. Financial Information
- 10.4.3. Biosimilar Pipeline
- 10.4.4. Recent Developments and Future Outlook
- 10.5. Intas Pharmaceuticals
- 10.5.1. Company Overview
- 10.5.2. Biosimilar Pipeline
- 10.5.3. Recent Developments and Future Outlook
- 10.6. Teva Pharmaceuticals
- 10.6.1. Company Overview
- 10.6.2. Financial Information
- 10.6.3. Biosimilar Pipeline
- 10.6.4. Recent Developments and Future Outlook
11. REGULATORY LANDSCAPE
- 11.1. Chapter Overview
- 11.2. Regulatory Guidelines for Biosimilars in North America
- 11.2.1. Regulatory Guidelines for Biosimilars in the US
- 11.2.1.1. Overview
- 11.2.1.2. Regulatory Landscape in the US
- 11.2.1.3. Regulatory Approval Pathway
- 11.2.1. Regulatory Guidelines for Biosimilars in the US
- 11.3. Regulatory Guidelines for Biosimilars in Europe
- 11.3.1. Overview
- 11.3.2. Regulatory Landscape in Europe
- 11.3.3. Regulatory Approval Pathway
- 11.4. Regulatory Guidelines for Biosimilars in Asia-Pacific
- 11.4.1. Regulatory Guidelines for Biosimilars in China
- 11.4.1.1. Overview
- 11.4.1.2. Regulatory Landscape in China
- 11.4.1.3. Regulatory Approval Pathway
- 11.4.2. Regulatory Guidelines for Biosimilars in Japan
- 11.4.2.1. Overview
- 11.4.2.2. Regulatory Landscape in Japan
- 11.4.2.3. Regulatory Approval Pathway
- 11.4.3. Regulatory Guidelines for Biosimilars in Australia
- 11.4.3.1. Overview
- 11.4.3.2. Regulatory Landscape in Australia
- 11.4.3.3. Regulatory Approval Pathway
- 11.4.1. Regulatory Guidelines for Biosimilars in China
- 11.5. Future Perspectives
12. COST PRICE ANALYSIS
- 12.1. Chapter Overview
- 12.2. Factors Contributing to High Price of Novel Biologics
- 12.3. Pricing of Biosimilars
- 12.3.1. Price Comparison of Different Biosimilars with its Reference Biologic
- 12.4. Concluding Remarks
13. CASE STUDY: OUTSOURCING OF BIOSIMILARS
- 13.1. Chapter Overview
- 13.2. Need for Outsourcing Manufacturing Operations
- 13.3. Impact of Biosimilars on the Global Contract Manufacturing Market
- 13.3.1. Biosimilars: Historical Trend of FDA Approvals
- 13.4. Biosimilars Contract Manufacturing Service Providers
- 13.5. Challenges Associated with Outsourcing of Biosimilar Manufacturing Operations
- 13.6. Future Perspectives
14. MARKET IMPACT ANALYSIS
- 14.1. Chapter Overview
- 14.2. Market Drivers
- 14.3. Market Restraints
- 14.4. Market Opportunities
- 14.5. Market Challenges
- 14.6. Conclusion
15. GLOBAL BIOSIMILARS MARKET
- 15.1. Chapter Overview
- 15.2. Key Assumptions and Methodology
- 15.3. Global Biosimilars Market, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 15.3.1. Scenario Analysis
- 15.3.1.1. Conservative Scenario
- 15.3.1.2. Optimistic Scenario
- 15.3.1. Scenario Analysis
- 15.4. Key Market Segmentations
16. BIOSIMILARS MARKET, BY DRUG CLASS
- 16.1. Chapter Overview
- 16.2. Key Assumptions and Methodology
- 16.3. Biosimilars Market: Distribution by Drug Class, 2019, 2024 and 2035
- 16.3.1. Biosimilars Market for Monoclonal Antibodies, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 16.3.2. Biosimilars Market for Proteins, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 16.3.3. Biosimilars Market for Peptides, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 16.3.4. Biosimilars Market for Others, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 16.4. Data Triangulation and Validation
17. BIOSIMILARS MARKET, BY THERAPEUTIC AREAS
- 17.1. Chapter Overview
- 17.2. Key Assumptions and Methodology
- 17.3. Biosimilars Market: Distribution by Therapeutic Areas, 2019, 2024 and 2035
- 17.3.1. Biosimilars Market for Oncological Disorders, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 17.3.2. Biosimilars Market for Autoimmune and Inflammatory Disorders, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 17.3.3. Biosimilars Market for Hematological Disorders, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 17.3.4. Biosimilars Market for Metabolic Disorders, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 17.3.5. Biosimilars Market for Other Disorders, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 17.4. Data Triangulation and Validation
18. BIOSIMILARS MARKET, BY TYPE OF MANUFACTURER
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. Biosimilars Market: Distribution by Type of Manufacturers, 2019, 2024 and 2035
- 18.3.1. Biosimilars Market for Contract Manufacturers, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 18.3.2. Biosimilars Market for In-house Developers, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 18.4. Data Triangulation and Validation
19. BIOSIMILARS MARKET, BY DISTRIBUTION CHANNELS
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Biosimilars Market: Distribution by Distribution Channels, 2019, 2024 and 2035
- 19.3.1. Biosimilars Market for Hospital Pharmacies, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 19.3.2. Biosimilars Market for Retail Pharmacies, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 19.3.3. Biosimilars Market for Online Pharmacies, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 19.4. Data Triangulation and Validation
20. BIOSIMILARS MARKET, BY GEOGRAPHICAL REGIONS
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Biosimilars Market: Distribution by Geographical Regions, 2019, 2024 and 2035
- 20.3.1. Biosimilars Market in North America, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.1.1. Biosimilars Market in the US, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.1.2. Biosimilars Market in Canada, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2. Biosimilars Market in Europe, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.1. Biosimilars Market in Germany, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.2. Biosimilars Market in the UK, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.3. Biosimilars Market in France, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.4. Biosimilars Market in Spain, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.5. Biosimilars Market in Italy, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.6. Biosimilars Market in Switzerland, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.7. Biosimilars Market in Belgium, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.8. Biosimilars Market in Denmark, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.2.9. Biosimilars Market in Rest of Europe, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.3. Biosimilars Market in Asia-Pacific, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.3.1. Biosimilars Market in China, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.3.2. Biosimilars Market in India, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.3.3. Biosimilars Market in Japan, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.3.4. Biosimilars Market in South Korea, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.3.5. Biosimilars Market in Australia, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.4. Biosimilars Market in Middle East and North Africa, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.4.1. Biosimilars Market in Saudi Arabia, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.4.2. Biosimilars Market in Egypt, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.4.3. Biosimilars Market in UAE, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.5. Biosimilars Market in Latin America, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.5.1. Biosimilars Market in Brazil, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.5.2. Biosimilars Market in Mexico, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.5.3. Biosimilars Market in Argentina, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.3.1. Biosimilars Market in North America, Historical Trends (since 2019) and Forecasted Estimates (till 2035)
- 20.4. Market Movement Analysis
- 20.5. Penetration-Growth (P-G) Matrix
- 20.6. Data Triangulation and Validation
21. BIOSIMILARS MARKET, BY LEADING PLAYERS
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology



















