
|
시장보고서
상품코드
1616874
의료기기 CRO 시장 - 치료 분야별, 사업 규모별, 기기 종류별, 임상 서비스 제공 유형별, 전임상 서비스 제공 유형별, 지역별 : 산업 동향 및 세계 예측(-2035년)Medical Device CRO Market by Target Therapeutic Area, Scale of Operation, Device Class, Type of Clinical Service Offered, Type of Preclinical Service Offered and Key Geographies : Industry Trends and Global Forecasts, Till 2035 |
||||||
세계 의료기기 CRO 시장 규모는 2035년까지 예측 기간 동안 3.4%의 CAGR로 확대되어 현재 133억 달러에서 2035년까지 193억 달러로 성장할 것으로 예상됩니다.
노인 인구의 증가와 만성질환을 중심으로 한 질병 발생으로 인해 여러 치료 분야에 걸쳐 새로운 의료기기에 대한 수요가 급증하고 있으며, 2020년 이후 미국 FDA는 약 105개의 의료기기를 승인했습니다. 또한, 매년 35개 이상의 의료기기가 FDA의 승인을 받고 있다는 점은 흥미롭습니다. 그러나 업계가 직면한 주요 과제 중 하나는 새로운 의료기기의 제품 개발 라이프사이클이 길고 복잡하다는 것입니다. 특히 임상 단계는 많은 자원이 필요하고 높은 비용과 큰 위험을 수반합니다. 또한, 의료기기의 안전성을 보장하기 위한 규제 지침이 엄격해져 업계 리더들에게 더 많은 어려움을 안겨주고 있습니다.
상기의 병목현상을 해결하기 위해 의료기기 개발 기업들은 의료기기 전문 CRO에 연구 업무를 적극적으로 아웃소싱하고 있습니다. CRO는 제조업체와 긴밀하게 협력하여 제품 개발 라이프사이클 전반을 엄격하게 모니터링하면서 참신한 아이디어를 시장 출시 가능한 제품으로 전환할 수 있도록 지원하며, 임상시험 수행, 규제 요건 탐색, 안전 기준 준수에 대한 전문 지식을 제공합니다. 이와 더불어 위험 모니터링 도구, 고급 데이터 분석, 리얼월드 증거 솔루션, 클라우드 컴퓨팅과 같은 첨단 기술과 도구의 통합으로 의료기기의 환경은 빠르게 변화하고 있습니다. 현재 진행 중인 연구개발과 의료기기 시장과 관련된 성장 기회의 증가로 인해 의료기기 CRO 시장은 향후 10년간 건전한 성장을 이룰 것으로 예상됩니다.
이 보고서는 세계 의료기기 CRO 시장을 조사하여 시장 개요와 함께 대상 치료 분야별, 사업 규모별, 기기 종류별, 임상 서비스 제공 유형별, 전임상 서비스 제공 유형별, 지역별 동향, 시장 진입 기업 프로파일 등을 제공합니다.
목차
제1장 서문
제2장 주요 요약
제3장 소개
제4장 시장 상황
제5장 의료기기 규제와 상환 상황
제6장 기업 개요
- 분석 개요
- Avania(Formerly known as Factory CRO)
- Charles River Laboratories
- CROMSOURCE
- CSSi LifeSciences
- Eurofins Medical Device Testing
- IQVIA
- Medpace
- NAMSA
- Qserve Group
- WuXi AppTec
제7장 의료기기 개발업체와 CRO의 관계 : 주요 촉진요인과 퍼포먼스 지표
제8장 경쟁 벤치마킹
제9장 주요 업계 브랜드 포지셔닝
- 분석 개요
- 범위와 조사 방법
- 브랜드 포지셔닝 매트릭스 : Labcorp
- 브랜드 포지셔닝 매트릭스 : IQVIA
- 브랜드 포지셔닝 매트릭스 : Syneos Health
- 브랜드 포지셔닝 매트릭스 : PPD
- 브랜드 포지셔닝 매트릭스 : ICON
- 브랜드 포지셔닝 매트릭스 : Charles River Laboratories
- 브랜드 포지셔닝 매트릭스 : WuXi AppTec
- 브랜드 포지셔닝 매트릭스 : Medpace
- 브랜드 포지셔닝 매트릭스 : NAMSA
제10장 임상시험 분석
제11장 인수합병
제12장 의료기기 임상시험수탁기관 총소유비용
제13장 조사 인사이트
제14장 시장 규모 평가와 기회 분석
제15장 SWOT 분석
제16장 향후 동향과 기회
제17장 결론
제18장 인터뷰 기록
제19장 부록 1 : 표형식 데이터
제20장 부록 2 : 기업·단체 리스트
ksm 24.12.30MEDICAL DEVICE CRO MARKET: OVERVIEW
As per Roots Analysis, the global medical device CRO market is estimated to grow from USD 13.3 billion in the current year to USD 19.3 billion by 2035, at a CAGR of 3.4% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Scale of Operation
- Clinical
- Preclinical
Device Class
- Class 1
- Class 2
- Class 3
Type of Clinical Services Offered
- Clinical Trial Management
- Data Management
- Regulatory Affair Management
- Consulting
Type of Preclinical Services Offered
- Biocompatibility Testing
- Sterility
- Microbiology Testing
- Material Characterization
- Analytical Services
Target Therapeutic Area
- Cardiovascular Disorders
- CNS Disorders
- Metabolic Disorders
- Oncological Disorders
- Ophthalmological Disorders
- Orthopedic Disorders
- Pain Disorders
- Psychological Disorders
- Respiratory Disorders
- Others
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
MEDICAL DEVICE CRO MARKET: GROWTH AND TRENDS
The increase in geriatric population and disease incidences, primarily chronic diseases, has led to a surge in the demand for novel medical devices across multiple therapeutic areas. Since 2020, the USFDA has approved ~105 medical devices. Further, it is interesting to note that over 35 medical devices receive FDA approval annually. However, one of the primary challenges faced by this industry is the time-consuming and complex product development lifecycle of a new medical device. Specifically, the clinical stage is exceedingly resource intensive, pertaining to higher costs and greater risks. In addition, the stringent regulatory guidelines to ensure the safety of medical devices make it more difficult for industrial leaders.
In order to address the abovementioned bottleneck, medical device developers are actively outsourcing their research operations to specialized medical device CRO. These organizations offer expertise in conducting clinical trials, navigating regulatory requirements, and ensuring compliance with safety standards. By partnering closely with manufacturers, CROs support translating novel ideas into market-ready products while rigorously monitoring throughout the product development lifecycle. In addition to this, the landscape of medical devices is rapidly changing, driven by the integration of advanced technologies and tools, including risk monitoring tools, advanced data analytics, real world evidence solutions and cloud computing. Owing to the ongoing research and development, and increasing opportunities linked to the medical devices market, it is anticipated that the medical devices CRO market will witness healthy growth in the coming decade.
MEDICAL DEVICE CRO MARKET: KEY INSIGHTS
The report delves into the current state of the medical device CRO market and identifies potential growth opportunities within the industry. Some key findings from the report include:
1. More than 590 CROs currently claim to have the required capabilities to offer a wide range of research and analytical services for medical devices, across different scale of operations.
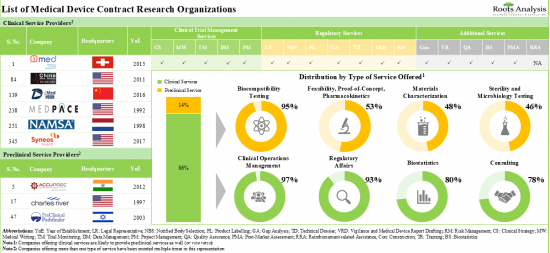
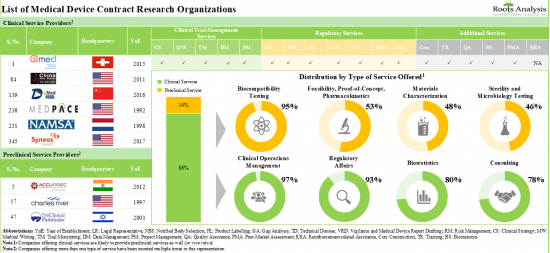
2. The market is well fragmented, featuring a mix of players based across different geographical regions that provide services for a range of medical device classes.
3. In pursuit of building a competitive edge, medical devices CROs are actively upgrading / expanding their capabilities to enhance their respective portfolios of offerings.
4. Over the years, industry players have undertaken a variety of initiatives to further advance the development / enable the improvement of their proprietary services for medical devices.
5. The rising interest in this domain is reflected in the number of mergers and acquisitions that have been reported in the last few years; 53% of such initiatives were focused on geographical consolidation.
6. Over 14,000 trials, enrolling close to 4.6 million patients suffering from a myriad of disease indications, have been registered across the globe to evaluate the efficacy and accuracy of medical devices.
7. Our proprietary total cost of ownership model suggests an informed estimate of direct and indirect expenses while setting up a contract research facility in different regions over a span of 20 years.
8. Driven by the ongoing efforts of industry stakeholders, the medical devices contract research service providers the market is expected to witness steady growth in the coming decade.
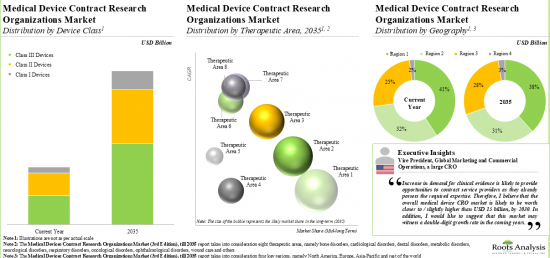
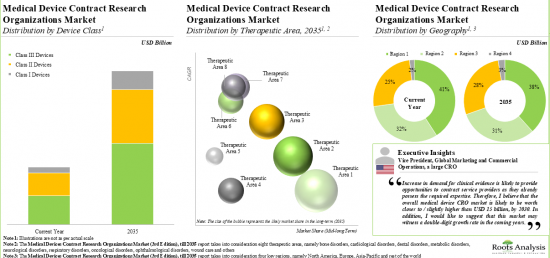
MEDICAL DEVICE CRO MARKET: KEY SEGMENTS
Currently, Clinical Operations Segment Occupies the Largest Share of the Medical Device CRO Market
Based on the scale of operation, the market is segmented into clinical and preclinical services. At present, the clinical services segment holds the maximum share of the medical device CRO market. It is worth highlighting that the increasing number of medical device-focused clinical trials is likely to drive the market in the near future.
Class II Devices Segment is the Fastest Growing Segment of the Medical Device CRO Market During the Forecast Period
Based on the device class, the market is segmented into Class I, Class II, Class III. Currently, class II devices hold maximum share within the medical devices CRO market. This trend is unlikely to change in the short-mid-term.
Currently, Clinical Trial Management Segment Occupies the Largest Share of the Medical Device CRO Market
Based on the type of clinical services, the market is segmented into clinical trial management, data management, regulatory affairs management, and consulting. At present, clinical trial management holds the maximum share of the medical devices CRO market. This trend is likely to remain the same in the forthcoming years.
Biocompatibility Testing Services Segment is the Fastest Growing Segment of the Medical Device CRO Market During the Forecast Period
Based on the type of preclinical services, the market is segmented into biocompatibility testing, sterility, microbiology testing, material characterization, and analytical services. It is worth highlighting that, at present, sterility and microbial testing holds a larger share of the medical device CRO market. This trend is likely to remain the same in the coming decade.
Currently, CNS Disorders Segment Occupies the Largest Share of the Medical Device CRO Market
Based on the therapeutic area, the market is segmented into cardiovascular disorders, CNS disorders, metabolic disorders, oncological disorders, ophthalmological disorders, orthopedic disorders, pain disorders, psychological disorders, respiratory disorders and other disorders. It is worth highlighting that majority of the current medical device CRO market is captured by CNS disorders.
North America Accounts for the Largest Share of the Market
Based on the key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and the Rest of the world. Majority share is expected to be captured by CROs based in North America. It is worth highlighting that, over the years, the market of the rest of the world is expected to grow at a higher CAGR.
Example Players in the Medical Device CRO Market
- Avania
- Charles River Laboratories
- CROMSOURCE
- CSSi LifeSciences
- Eurofins Medical Testing
- IQVIA
- Medpace
- NAMSA
- Qserve Group
- WuXi AppTec
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Chief Executive Officer and Co-founder, HungaroTrial
- Senior Vice President of Business Development and Marketing, ClinChoice
- Vice President of Global Marketing and Commercial Operations, NAMSA
- Head of Business Development, CTC North
- General Manager, CW Research & Management
- Chief Operating Officer, CROMSOURCE
- Senior manager, Medical & Clinical Operations, Metrics Research
- Technical Director and Partner, Vyomus Consulting
- Director of Business Development, A+ Science
- Business Development Manager, AtoZ-CRO
MEDICAL DEVICE CRO MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the medical device CRO market, focusing on key market segments, including [A] scale of operation, [B] device class, [C] type of clinical services offered, [D] type of preclinical services offered, [E] therapeutic area and [F] geographical regions.
- Market Landscape: A comprehensive evaluation of contract research organizations involved in the medical device CRO market, considering various parameters, such as [A] year of establishment, [B] company size (in terms of the number of employees), [C] location of headquarters, [D] area of specialization, [E] device class, [F] types of CRO services offered by [F1] clinical service providers, [F2] preclinical service providers and [F3] stand-alone service providers.
- Regulatory and Reimbursement Landscape Analysis: A discussion on general regulatory guidelines established and issued by major regulatory bodies for medical device approval. In addition, it includes an insightful multi-dimensional heat map analysis, featuring a comparison of the contemporary regulatory and reimbursement scenarios in key geographies across the globe.
- Company Profiles: In-depth profiles of key industry players offering CRO services for both clinical and preclinical stage development of medical devices, focusing on [A] company overviews, [B] medical device-focused service portfolio, [C] recent developments and [D] an informed future outlook.
- Competitive Benchmarking Analysis: A competitive benchmarking analysis that emphasizes the primary focus areas of small, mid-sized, and large companies. This analysis compares their existing capabilities within and beyond their respective peer groups based in North America, Europe, Asia-Pacific, and other regions. It offers stakeholders insights into potential strategies for achieving a competitive edge in the industry.
- Key Performance Indicators: An analysis highlighting the key performance indicators used by sponsor companies to evaluate service providers engaged in the medical device CRO market, based on information gathered via secondary research (for top-ten medical device players) and primary research.
- Brand Positioning Analysis: A comprehensive analysis of brand positioning for top industry players, highlighting the prevailing perceptions of their proprietary brands.
- Clinical Trials Analysis: Examination of completed, ongoing, and planned clinical studies of various medical devices, based on parameters like [A] trial registration year, [B] trial phase, [C] current trial status, [D] enrolled patient population, [E] study design, [F] leading industry players (in terms of number of trials conducted), [G] target therapeutic area(s) and [H] key geographical regions.
- Merger and Acquisitions: A comprehensive examination of the various mergers and acquisitions, focusing on multiple relevant parameters, including [A] year of agreement, [B] type of agreement and [C] geographical location of the companies.
- Total Cost of Ownership: An in-depth analysis of the total cost of ownership for a medical device contract research service provider, which includes a well-informed estimate of both direct and indirect costs.
- Survey Analysis: A survey analysis featuring inputs solicited from various experts who are directly / indirectly involved in providing CRO services to medical device developers.
- SWOT Analysis: A SWOT analysis, focusing on key drivers and challenges that are likely to impact the industry's evolution. Further, it includes a Harvey ball analysis, highlighting the relative effect of each SWOT parameter on the overall industry.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Key Questions Answered
- 1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview of Medical Devices
- 3.2.1. Historical Evolution of Medical Devices
- 3.2.2. Classification of Medical Devices
- 3.3. Overview of Contract Research Organizations (CROs)
- 3.3.1. Evolution of CROs
- 3.4. Role of CROs in the Medical Device Industry
- 3.5. Types of Medical Device CROs
- 3.6. Types of Services Offered by CROs
- 3.7. Advantages of Outsourcing Operations to CROs
- 3.8. Risks and Challenges Associated with Outsourcing
- 3.9. Key Considerations WhileSelecting a Suitable CRO Partner
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Medical Device CROs: Clinical Service Providers
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Company Size and Location of Headquarters
- 4.2.5. Analysis by Area of Specialization
- 4.2.6. Analysis by Device Class
- 4.2.7. Analysis by Type of Clinical Operation Service Offered
- 4.2.8. Analysis by Type of Regulatory Affairs-related Service Offered
- 4.2.9. Analysis by Type of Additional Service Offered
- 4.2.10. Analysis by Medical Device Regulatory Compliance Authority
- 4.3. Medical Device CROs: Preclinical Service Providers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters
- 4.3.4. Analysis by Company Size and Location of Headquarters
- 4.3.5. Analysis by Type of Preclinical Service Offered
- 4.4. Medical Device CROs: Standalone Service Providers
- 4.4.1. Analysis by Year of Establishment
- 4.4.2. Analysis by Company Size
- 4.4.3. Analysis by Location of Headquarters
- 4.4.4. Analysis by Company Size and Location of Headquarters
5. REGULATORY AND REIMBURSEMENT LANDSCAPE FOR MEDICAL DEVICES
- 5.1. Chapter Overview
- 5.2. General Regulatory and Reimbursement Guidelines for Medical Devices
- 5.3. Regulatory and Reimbursement Landscape in North America
- 5.3.1. The US Scenario
- 5.3.1.1. Regulatory Authority
- 5.3.1.2. Review / Approval Process
- 5.3.1.3. Reimbursement Landscape
- 5.3.1.3.1. Payer Mix
- 5.3.1.3.2. Reimbursement Process
- 5.3.2. The Canadian Scenario
- 5.3.2.1. Regulatory Authority
- 5.3.2.2. Review / Approval Process
- 5.3.2.3. Reimbursement Landscape
- 5.3.2.3.1. Payer Mix
- 5.3.2.3.2. Reimbursement Process
- 5.3.3. The Mexican Scenario
- 5.3.3.1. Regulatory Authority
- 5.3.3.2. Review / Approval Process
- 5.3.3.3. Reimbursement Landscape
- 5.3.3.3.1. Payer Mix
- 5.3.1. The US Scenario
- 5.4. Regulatory and Reimbursement Landscape in Europe
- 5.4.1. Overall Scenario
- 5.4.1.1. Overview of Regulatory Authorities
- 5.4.1.2. Overall Review / Approval Process
- 5.4.2. The UK Scenario
- 5.4.2.1. Regulatory Authority
- 5.4.2.2. Review / Approval Process
- 5.4.2.3. Reimbursement Landscape
- 5.4.2.3.1. Payer Mix
- 5.4.2.3.2. Reimbursement Process
- 5.4.3. The French Scenario
- 5.4.3.1. Regulatory Authority
- 5.4.3.2. Review / Approval Process
- 5.4.3.3. Reimbursement Landscape
- 5.4.3.3.1. Payer Mix
- 5.4.3.3.2. Reimbursement Process
- 5.4.4. The German Scenario
- 5.4.4.1. Regulatory Authority
- 5.4.4.2. Review / Approval Process
- 5.4.4.3. Reimbursement Landscape
- 5.4.4.3.1. Payer Mix
- 5.4.4.3.2. Reimbursement Process
- 5.4.5. The Italian Scenario
- 5.4.5.1. Regulatory Authority
- 5.4.5.2. Review / Approval Process
- 5.4.5.3. Reimbursement Landscape
- 5.4.5.3.1. Payer Mix
- 5.4.5.3.2. Reimbursement Process
- 5.4.6. The Spanish Scenario
- 5.4.6.1. Regulatory Authority
- 5.4.6.2. Review / Approval Process
- 5.4.6.3. Reimbursement Landscape
- 5.4.6.3.1. Payer Mix
- 5.4.6.3.2. Reimbursement Process
- 5.4.1. Overall Scenario
- 5.5. Regulatory and Reimbursement Landscape in Asia-Pacific and Rest of the World
- 5.5.1. The Australian Scenario
- 5.5.1.1. Regulatory Authority
- 5.5.1.2. Review / Approval Process
- 5.5.1.3. Reimbursement Landscape
- 5.5.1.3.1. Payer Mix
- 5.5.1.3.2. Reimbursement Process
- 5.5.2. The Brazilian Scenario
- 5.5.2.1. Regulatory Authority
- 5.5.2.2. Review / Approval Process
- 5.5.2.3. Reimbursement Landscape
- 5.5.2.3.1. Payer Mix
- 5.5.2.3.2. Reimbursement Process
- 5.5.3. The Chinese Scenario
- 5.5.3.1. Regulatory Authority
- 5.5.3.2. Review / Approval Process
- 5.5.3.3. Reimbursement Landscape
- 5.5.3.3.1. Payer Mix
- 5.5.3.3.2. Reimbursement Process
- 5.5.4. The Indian Scenario
- 5.5.4.1. Regulatory Authority
- 5.5.4.2. Review / Approval Process
- 5.5.4.3. Reimbursement Landscape
- 5.5.4.3.1. Payer Mix
- 5.5.5. The Israeli Scenario
- 5.5.5.1. Regulatory Authority
- 5.5.5.2. Review / Approval Process
- 5.5.5.3. Reimbursement Landscape
- 5.5.5.3.1. Payer Mix
- 5.5.6. The Japanese Scenario
- 5.5.6.1. Regulatory Authority
- 5.5.6.2. Review / Approval Process
- 5.5.6.3. Reimbursement Landscape
- 5.5.6.3.1. Payer Mix
- 5.5.6.3.2. Reimbursement Process
- 5.5.7. The New Zealand Scenario
- 5.5.7.1. Regulatory Authority
- 5.5.7.2. Review / Approval Process
- 5.5.7.3. Reimbursement Landscape
- 5.5.7.3.1. Payer Mix
- 5.5.7.3.2. Reimbursement Process
- 5.5.8. The Singapore Scenario
- 5.5.8.1. Regulatory Authority
- 5.5.8.2. Review / Approval Process
- 5.5.8.3. Reimbursement Landscape
- 5.5.8.3.1. Payer Mix
- 5.5.8.3.2. Reimbursement Process
- 5.5.9. The South Korean Scenario
- 5.5.9.1. Regulatory Authority
- 5.5.9.2. Review / Approval Process
- 5.5.9.3. Reimbursement Landscape
- 5.5.9.3.1. Payer Mix
- 5.5.9.3.2. Reimbursement Process
- 5.5.10. The South African Scenario
- 5.5.10.1. Regulatory Authority
- 5.5.10.2. Review / Approval Process
- 5.5.10.3. Reimbursement Landscape
- 5.5.11. The Taiwan Scenario
- 5.5.11.1. Regulatory Authority
- 5.5.11.2. Review / Approval Process
- 5.5.11.3. Reimbursement Landscape
- 5.5.11.3.1. Payer Mix
- 5.5.11.3.2. Reimbursement Process
- 5.5.12. The Thailand Scenario
- 5.5.12.1. Regulatory Authority
- 5.5.12.2. Review / Approval Process
- 5.5.12.3. Reimbursement Landscape
- 5.5.1. The Australian Scenario
- 5.6. Comparison of Regional Regulatory Control
- 5.7. Concluding Remarks
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Avania (Formerly known as Factory CRO)
- 6.2.1. Company Overview
- 6.2.2. Service Portfolio
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Charles River Laboratories
- 6.3.1. Company Overview
- 6.3.2. Service Portfolio
- 6.3.3. Recent Developments and Future Outlook
- 6.4. CROMSOURCE
- 6.4.1. Company Overview
- 6.4.2. Service Portfolio
- 6.4.3. Recent Developments and Future Outlook
- 6.5. CSSi LifeSciences
- 6.5.1. Company Overview
- 6.5.2. Service Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. Eurofins Medical Device Testing
- 6.6.1. Company Overview
- 6.6.2. Service Portfolio
- 6.6.3. Recent Developments and Future Outlook
- 6.7. IQVIA
- 6.7.1. Company Overview
- 6.7.2. Service Portfolio
- 6.7.3. Recent Developments and Future Outlook
- 6.8. Medpace
- 6.8.1. Company Overview
- 6.8.2. Service Portfolio
- 6.8.3. Recent Developments and Future Outlook
- 6.9. NAMSA
- 6.9.1. Company Overview
- 6.9.2. Service Portfolio
- 6.9.3. Recent Developments and Future Outlook
- 6.10. Qserve Group
- 6.10.1. Company Overview
- 6.10.2. Service Portfolio
- 6.10.3. Recent Developments and Future Outlook
- 6.11. WuXi AppTec
- 6.11.1. Company Overview
- 6.11.2. Service Portfolio
- 6.11.3. Recent Developments and Future Outlook
7. MEDICAL DEVICE DEVELOPER AND CRO RELATIONSHIPS: KEY VALUE DRIVERS AND PERFORMANCE INDICATORS
- 7.1. Chapter Overview
- 7.2. Definition and Importance of Key Performance Indicators (KPIs)
- 7.3. Key Considerations for Selection of KPIs
- 7.4. Types of KPIs
- 7.4.1. Financial Indicators
- 7.4.1.1. Most Important KPIs
- 7.4.1.1.1. Financial Stability
- 7.4.1.1.2. Cost of Services Offered
- 7.4.1.1.3. Comparative Analysis of Financial Indicators
- 7.4.1.2. Industry Perspective
- 7.4.1.2.1. Sponsors' (Big Pharma) Perspective
- 7.4.1.2.2. Contract Service Providers' Perspective
- 7.4.1.1. Most Important KPIs
- 7.4.2. Process and Capability Indicators
- 7.4.2.1. Most Important KPIs
- 7.4.2.1.1. Proximity to Sponsor
- 7.4.2.1.2. Capability to Innovate / Mitigate Risk
- 7.4.2.1.3. Strength of Service Portfolio
- 7.4.2.1.4. Comparative Analysis of Process / Capability Indicators
- 7.4.2.2. Industry Perspective
- 7.4.2.2.1. Sponsors' (Big Pharma) Perspective
- 7.4.2.2.2. Contract Service Providers' Perspective
- 7.4.2.1. Most Important KPIs
- 7.4.3. Market Reputation Indicators
- 7.4.3.1. Most Important KPIs
- 7.4.3.1.1. Flexibility / Adaptability
- 7.4.3.1.2. Time Management
- 7.4.3.1.3. Quality / Reliability
- 7.4.3.1.4. Regulatory Compliance / Track Record
- 7.4.3.1.5. Comparative Analysis of Market Reputation Indicators
- 7.4.3.2. Industry Perspective
- 7.4.3.2.1. Sponsors' (Big Pharma) Perspective
- 7.4.3.2.2. Contract Service Providers' Perspective
- 7.4.3.1. Most Important KPIs
- 7.4.1. Financial Indicators
- 7.5. Comparison of Key Performance Indicators
- 7.6. Concluding Remarks
8. COMPETITIVE BENCHMARKING
- 8.1. Chapter Overview
- 8.2. Assumptions and Methodology
- 8.3. Competitive Benchmarking by Region
- 8.3.1. Competitive Benchmarking: Small Players based in North America (Peer Group I)
- 8.3.2. Competitive Benchmarking: Mid-sized Players based in North America (Peer Group II)
- 8.3.3. Competitive Benchmarking: Large Players based in North America (Peer Group III)
- 8.3.4. Competitive Benchmarking: Small Players based in Europe (Peer Group IV)
- 8.3.5. Competitive Benchmarking: Mid-sized Players based in Europe (Peer Group V)
- 8.3.6. Competitive Benchmarking: Large Players based in Europe (Peer Group VI)
- 8.3.7. Competitive Benchmarking: Small Players based in Asia-Pacific (Peer Group VII)
- 8.3.8. Competitive Benchmarking: Mid-sized Players based in Asia-Pacific (Peer Group VIII)
- 8.3.9. Competitive Benchmarking: Large Players based in Asia-Pacific (Peer Group IX)
- 8.3.10. Competitive Benchmarking: Small Players based in RoW (Peer Group X)
- 8.3.11. Competitive Benchmarking: Mid-sized Players based in RoW (Peer Group XI)
- 8.4. Concluding Remarks
9. BRAND POSITIONING OF KEY INDUSTRY PLAYERS
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.3. Brand Positioning Matrix: Labcorp
- 9.4. Brand Positioning Matrix: IQVIA
- 9.5. Brand Positioning Matrix: Syneos Health
- 9.6. Brand Positioning Matrix: PPD
- 9.7. Brand Positioning Matrix: ICON
- 9.8. Brand Positioning Matrix: Charles River Laboratories
- 9.9. Brand Positioning Matrix: WuXi AppTec
- 9.10. Brand Positioning Matrix: Medpace
- 9.11. Brand Positioning Matrix: NAMSA
10. CLINICAL TRIAL ANALYSIS
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Medical Devices: Clinical Trial Analysis
- 10.3.1. Analysis by Trial Registration Year
- 10.3.2. Analysis by Trial Status
- 10.3.3. Analysis by Phase of Development
- 10.3.4. Analysis by Study Design
- 10.3.5. Analysis by Therapeutic Area
- 10.3.6. Analysis by Geography
- 10.3.7. Analysis by Trial Registration Year and Geography
- 10.3.8. Analysis by Trial Status and Geography
- 10.3.9. Analysis by Type of Sponsor
- 10.3.10. Most Active Players: Analysis by Number of Clinical Trials
- 10.4. Medical Devices: Analysis by Enrolled Patient Population
- 10.4.1. Analysis by Trial Registration Year
- 10.4.2. Analysis by Phase of Development
- 10.4.3. Analysis by Geography
- 10.4.4. Analysis by Trial Status and Geography
11. MERGERS AND ACQUISITIONS
- 11.1. Chapter Overview
- 11.2. Merger and Acquisition Models
- 11.3. Medical Device CROs: Mergers and Acquisitions
- 11.3.1. Analysis by Year of Merger / Acquisition
- 11.3.2. Analysis by Type of Agreement
- 11.3.3. Regional Analysis
- 11.3.3.1. Analysis by Continent
- 11.3.3.2. Intercontinental and Intracontinental Deals
- 11.3.3.3. Analysis by Country
- 11.3.4. Ownership Change Matrix
- 11.3.5. Analysis by Type of Company
- 11.3.6. Analysis by Key Value Drivers
- 11.3.6.1. Analysis by Year of Acquisition and Key Value Drivers
12. TOTAL COST OF OWNERSHIP FOR MEDICAL DEVICES CONTRACT RESEARCH ORGANIZATION
- 12.1. Chapter Overview
- 12.2. Key Parameters
- 12.3. Assumptions and Methodology
- 12.4. Sample Dataset for the estimation of Total Cost of Ownership
- 12.4.1. Total Cost of Ownership for Large Medical Device Contract Research Organizations, Y0-Y20
- 12.4.2. Total Cost of Ownership for Large Medical Device Contract Research Organizations: Analysis by CAPEX, Y0
- 12.4.3. Total Cost of Ownership for Large Medical Device Contract Research Organizations: Analysis by OPEX, Y1-Y20
13. SURVEY INSIGHTS
- 13.1. Chapter Overview
- 13.2. Designation of Respondents
- 13.3. Types of Services Offered
- 13.4. Target Therapeutic Area
- 13.5. Average Number of Annual Projects
- 13.6. Market Opportunity
14. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 14.1. Chapter Overview
- 14.2. Assumptions and Forecast Methodology
- 14.3. Overall Medical Device CROs Market, till 2035
- 14.3.1. Medical Device CROs Market: Analysis by Therapeutic Area, 2022, 2028 and 2035
- 14.3.2. Medical Device CROs Market: Analysis by Scale of Operation, till 2035
- 14.3.3. Medical Device CROs Market: Analysis by Device Class, till 2035
- 14.3.4. Medical Device CROs Market, till 2035: Analysis by Type of Clinical Service Offered, till 2035
- 14.3.5. Medical Device CROs Market, till 2035: Analysis by Type of Preclinical Service Offered, till 2035
- 14.3.6. Medical Device CROs Market: Analysis by Geography, 2022, 2028 and 2035
- 14.4. Medical Device CROs Market in North America, till 2035
- 14.4.1. Medical Device CROs Market in North America: Analysis by Therapeutic Area, till 2035
- 14.4.1.1. Medical Device CROs Market for CNS Disorders in North America, till 2035
- 14.4.1.2. Medical Device CROs Market for Cardiovascular Disorders in North America, till 2035
- 14.4.1.3. Medical Device CROs Market for Oncological Disorders in North America, till 2035
- 14.4.1.4. Medical Device CROs Market for Bone Disorders in North America, till 2035
- 14.4.1.5. Medical Device CROs Market for Respiratory Disorders in North America, till 2035
- 14.4.1.6. Medical Device CROs Market for Pain Management Disorders in North America, till 2035
- 14.4.1.7. Medical Device CROs Market for Ophthalmic Disorders in North America, till 2035
- 14.4.1.8. Medical Device CROs Market for Psychological Disorders in North America, till 2035
- 14.4.1.9. Medical Device CROs Market for Metabolic Disorders in North America, till 2035
- 14.4.1.10. Medical Device CROs Market for Other Disorders in North America, till 2035
- 14.4.2. Medical Device CROs Market in North America: Analysis by Device Class, till 2035
- 14.4.2.1. Medical Device CROs Market for Class I Devices in North America, till 2035
- 14.4.2.2. Medical Device CROs Market for Class II Devices in North America, till 2035
- 14.4.2.3. Medical Device CROs Market for Class III Devices in North America, till 2035
- 14.4.3. Medical Device CROs Market in North America: Analysis by Type of Clinical Service Offered, till 2035
- 14.4.3.1. Medical Device CROs Market for Clinical Trial Management Services in North America, till 2035
- 14.4.3.2. Medical Device CROs Market for Consulting Services in North America, till 2035
- 14.4.3.3. Medical Device CROs Market for Data Management Services in North America, till 2035
- 14.4.3.4. Medical Device CROs Market for Regulatory Affairs Management Services in North America, till 2035
- 14.4.3.5. Medical Device CROs Market for Other Clinical Services in North America, till 2035
- 14.4.4. Medical Device CROs Market in North America: Analysis by Type of Preclinical Service Offered, till 2035
- 14.4.4.1. Medical Device CROs Market for Material Characterization and Analytical Services in North America, till 2035
- 14.4.4.2. Medical Device CROs Market for Biocompatibility Testing Services in North America, till 2035
- 14.4.4.3. Medical Device CROs Market for Sterility and Microbiology Testing Services in North America, till 2035
- 14.4.4.4. Medical Device CROs Market for Other Preclinical Services in North America, till 2035
- 14.4.1. Medical Device CROs Market in North America: Analysis by Therapeutic Area, till 2035
- 14.5. Medical Device CROs Market in Europe, till 2035
- 14.5.1. Medical Device CROs Market in Europe: Analysis by Therapeutic Area, till 2035
- 14.5.1.1. Medical Device CROs Market for CNS Disorders in Europe, till 2035
- 14.5.1.2. Medical Device CROs Market for Cardiovascular Disorders in Europe, till 2035
- 14.5.1.3. Medical Device CROs Market for Oncological Disorders in Europe, till 2035
- 14.5.1.4. Medical Device CROs Market for Bone Disorders in Europe, till 2035
- 14.5.1.5. Medical Device CROs Market for Respiratory Disorders in Europe, till 2035
- 14.5.1.6. Medical Device CROs Market for Pain Management Disorders in Europe, till 2035
- 14.5.1.7. Medical Device CROs Market for Ophthalmic Disorders in Europe, till 2035
- 14.5.1.8. Medical Device CROs Market for Psychological Disorders in Europe, till 2035
- 14.5.1.9. Medical Device CROs Market for Metabolic Disorders in Europe, till 2035
- 14.5.1.10. Medical Device CROs Market for Other Disorders in Europe, till 2035
- 14.5.2. Medical Device CROs Market in Europe: Analysis by Device Class, till 2035
- 14.5.2.1. Medical Device CROs Market for Class I Devices in Europe, till 2035
- 14.5.2.2. Medical Device CROs Market for Class II Devices in Europe, till 2035
- 14.5.2.3. Medical Device CROs Market for Class III Devices in Europe, till 2035
- 14.5.3. Medical Device CROs Market in Europe: Analysis by Type of Clinical Service Offered, till 2035
- 14.5.3.1. Medical Device CROs Market for Clinical Trial Management Services in Europe, till 2035
- 14.5.3.2. Medical Device CROs Market for Consulting Services in Europe, till 2035
- 14.5.3.3. Medical Device CROs Market for Data Management Services in Europe, till 2035
- 14.5.3.4. Medical Device CROs Market for Regulatory Affairs Management Services in Europe, till 2035
- 14.5.3.5. Medical Device CROs Market for Other Clinical Services in Europe, till 2035
- 14.5.4. Medical Device CROs Market in Europe: Analysis by Type of Preclinical Service Offered, till 2035
- 14.5.4.1. Medical Device CROs Market for Material Characterization and Analytical Services in Europe, till 2035
- 14.5.4.2. Medical Device CROs Market for Biocompatibility Testing Services in Europe, till 2035
- 14.5.4.3. Medical Device CROs Market for Sterility and Microbiology Testing Services in Europe, till 2035
- 14.5.4.4. Medical Device CROs Market for Other Preclinical Services in Europe, till 2035
- 14.5.1. Medical Device CROs Market in Europe: Analysis by Therapeutic Area, till 2035
- 14.6. Medical Device CROs Market in Asia-Pacific, till 2035
- 14.6.1. Medical Device CROs Market in Asia-Pacific: Analysis by Therapeutic Area, till 2035
- 14.6.1.1. Medical Device CROs Market for CNS Disorders in Asia-Pacific, till 2035
- 14.6.1.2. Medical Device CROs Market for Cardiovascular Disorders in Asia-Pacific, till 2035
- 14.6.1.3. Medical Device CROs Market for Oncological Disorders in Asia-Pacific, till 2035
- 14.6.1.4. Medical Device CROs Market for Bone Disorders in Asia-Pacific, till 2035
- 14.6.1.5. Medical Device CROs Market for Respiratory Disorders in Asia-Pacific, till 2035
- 14.6.1.6. Medical Device CROs Market for Pain Management Disorders in Asia-Pacific, till 2035
- 14.6.1.7. Medical Device CROs Market for Ophthalmic Disorders in Asia-Pacific, till 2035
- 14.6.1.8. Medical Device CROs Market for Psychological Disorders in Asia-Pacific, till 2035
- 14.6.1.9. Medical Device CROs Market for Metabolic Disorders in Asia-Pacific, till 2035
- 14.6.1.10. Medical Device CROs Market for Other Disorders in Asia-Pacific, till 2035
- 14.6.2. Medical Device CROs Market in Asia-Pacific: Analysis by Device Class, till 2035
- 14.6.2.1. Medical Device CROs Market for Class I Devices in Asia-Pacific, till 2035
- 14.6.2.2. Medical Device CROs Market for Class II Devices in Asia-Pacific, till 2035
- 14.6.2.3. Medical Device CROs Market for Class III Devices in Asia-Pacific, till 2035
- 14.6.3. Medical Device CROs Market in Asia-Pacific: Analysis by Type of Clinical Service Offered, till 2035
- 14.6.3.1. Medical Device CROs Market for Clinical Trial Management Services in Asia-Pacific, till 2035
- 14.6.3.2. Medical Device CROs Market for Consulting Services in Asia-Pacific, till 2035
- 14.6.3.3. Medical Device CROs Market for Data Management Services in Asia-Pacific, till 2035
- 14.6.3.4. Medical Device CROs Market for Regulatory Affairs Management Services in Asia-Pacific, till 2035
- 14.6.3.5. Medical Device CROs Market for Other Clinical Services in Asia-Pacific, till 2035
- 14.6.4. Medical Device CROs Market in Asia-Pacific: Analysis by Type of Preclinical Service Offered, till 2035
- 14.6.4.1. Medical Device CROs Market for Material Characterization and Analytical Services in Asia-Pacific, till 2035
- 14.6.4.2. Medical Device CROs Market for Biocompatibility Testing Services in Asia-Pacific, till 2035
- 14.6.4.3. Medical Device CROs Market for Sterility and Microbiology Testing Services in Asia-Pacific, till 2035
- 14.6.4.4. Medical Device CROs Market for Other Preclinical Services in Asia-Pacific, till 2035
- 14.6.1. Medical Device CROs Market in Asia-Pacific: Analysis by Therapeutic Area, till 2035
- 14.7. Medical Device CROs Market in Rest of the World, till 2035
- 14.7.1. Medical Device CROs Market in Rest of the World: Analysis by Therapeutic Area, till 2035
- 14.7.1.1. Medical Device CROs Market for CNS Disorders in Rest of the World, till 2035
- 14.7.1.2. Medical Device CROs Market for Cardiovascular Disorders in Rest of the World, till 2035
- 14.7.1.3. Medical Device CROs Market for Oncological Disorders in Rest of the World, till 2035
- 14.7.1.4. Medical Device CROs Market for Bone Disorders in Rest of the World, till 2035
- 14.7.1.5. Medical Device CROs Market for Respiratory Disorders in Rest of the World, till 2035
- 14.7.1.6. Medical Device CROs Market for Pain Management Disorders in Rest of the World, till 2035
- 14.7.1.7. Medical Device CROs Market for Ophthalmic Disorders in Rest of the World, till 2035
- 14.7.1.8. Medical Device CROs Market for Psychological Disorders in Rest of the World, till 2035
- 14.7.1.9. Medical Device CROs Market for Metabolic Disorders in Rest of the World, till 2035
- 14.7.1.10. Medical Device CROs Market for Other Disorders in Rest of the World, till 2035
- 14.7.2. Medical Device CROs Market in Rest of the World: Analysis by Device Class, till 2035
- 14.7.2.1. Medical Device CROs Market for Class I Devices in Rest of the World, till 2035
- 14.7.2.2. Medical Device CROs Market for Class II Devices in Rest of the World, till 2035
- 14.7.2.3. Medical Device CROs Market for Class III Devices in Rest of the World, till 2035
- 14.7.3. Medical Device CROs Market in Rest of the World: Analysis by Type of Clinical Service Offered, till 2035
- 14.7.3.1. Medical Device CROs Market for Clinical Trial Management Services in Rest of the World, till 2035
- 14.7.3.2. Medical Device CROs Market for Consulting Services in Rest of the World, till 2035
- 14.7.3.3. Medical Device CROs Market for Data Management Services in Rest of the World, till 2035
- 14.7.3.4. Medical Device CROs Market for Regulatory Affairs Management Services in Rest of the World, till 2035
- 14.7.3.5. Medical Device CROs Market for Other Clinical Services in Rest of the World, till 2035
- 14.7.4. Medical Device CROs Market in Rest of the World: Analysis by Type of Preclinical Service Offered, till 2035
- 14.7.4.1. Medical Device CROs Market for Material Characterization and Analytical Services in Rest of the World, till 2035
- 14.7.4.2. Medical Device CROs Market for Biocompatibility Testing Services in Rest of the World, till 2035
- 14.7.4.3. Medical Device CROs Market for Sterility and Microbiology Testing Services in Rest of the World, till 2035
- 14.7.4.4. Medical Device CROs Market for Other Preclinical Services in Rest of the World, till 2035
- 14.7.1. Medical Device CROs Market in Rest of the World: Analysis by Therapeutic Area, till 2035
15. SWOT ANALYSIS
- 15.1. Chapter Overview
- 15.2. Strengths
- 15.3. Weaknesses
- 15.4. Opportunities
- 15.5. Threats
- 15.6. Concluding Remarks
16. FUTURE TRENDS AND OPPORTUNITIES
- 16.1. Chapter Overview
- 16.2. Anticipated Growth in Number of Connected Devices
- 16.3. Adoption of Data Analytics and Real-Time Monitoring Solutions
- 16.4. Need for Cloud-based Computing and Storage Solutions
- 16.5. Growing Demand for Real World Evidence to Obtain Regulatory Approval
- 16.6. Concluding Remarks
17. CONCLUDING REMARKS
18. INTERVIEW TRANSCRIPTS
- 18.1. Chapter Overview
- 18.2. HungaroTrial
- 18.2.1. Company Snapshot
- 18.2.2. Interview Transcript: Lajos Sarosi, Chief Executive Officer and Co-founder
- 18.3. ClinChoice
- 18.3.1. Company Snapshot
- 18.3.2. Lee King, Senior Vice President of Business Development and Marketing
- 18.4. NAMSA
- 18.4.1. Company Snapshot
- 18.4.2. Christopher Rupp, Vice President of Global Marketing and Commercial Operations
- 18.5. CTC North
- 18.5.1. Company Snapshot
- 18.5.2. Claus Hemiker, Head of Business Development
- 18.6. CW Research & Management
- 18.6.1. Company Snapshot
- 18.6.2. Christian Wolflehner, General Manager
- 18.7. CROMSOURCE
- 18.7.1. Company Snapshot
- 18.7.2. Troy McCall, Chief Operating Officer
- 18.8. Metrics Research
- 18.8.1. Company Snapshot
- 18.8.2. Nazish Urooj, Senior manager, Medical & Clinical Operations
- 18.9. Vyomus Consulting
- 18.9.1. Company Snapshot
- 18.9.2. C. Omprakash, Technical Director and Partner
- 18.10. A+ Science
- 18.10.1. Company Snapshot
- 18.10.2. Tania Persson, Director of Business Development
- 18.11. AtoZ-CRO
- 18.11.1. Company Snapshot
- 18.11.2. Alexa Foltin-Mertgen, Business Development Manager











