
|
시장보고서
상품코드
1648561
휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장 : 스텝별, 조사 방법 유형별, 테스트 대상 의료기기 클래스별, 지역별, 주요 기업별 - 업계 동향과 세계 예측(-2035년)Human Factors and Usability Engineering Services Market by Steps Involved, Type of Research Method, Class of Medical Device Tested, Geographical Regions and Leading Players: Industry Trends and Global Forecasts, Till 2035 |
||||||
세계의 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장 규모는 2035년까지의 예측 기간 중 6.9%의 CAGR로 확대하며, 현재 9억 4,000만 달러에서 2035년까지 19억 7,000만 달러로 성장할 것으로 예측됩니다.
수년 동안 휴먼팩터와 사용성 엔지니어링은 모든 유형의 의료기기 개발에 필수적인 요소로 자리 잡았습니다. 그 결과, 개발자들은 기기의 안전성, 유효성 및 사용자 경험을 향상시키기 위해 개발중인 기기에 대한 휴먼팩터 연구를 수행하는 서비스 프로바이더에 적극적으로 의존하고 있습니다. 의료기기가 점점 더 복잡해짐에 따라 사용시 발생할 수 있는 잠재적 위험을 최소화하기 위해 프로토타입과 사용자 인터페이스 설계에 집중하는 것이 필수적입니다. 또한 규제기관(특히 미국 식품의약국(USFDA)은 의료기기 설계 및 평가에 인적 요소의 통합을 점점 더 많이 요구하고 있습니다. 이들 기관은 제조업체가 사용성 공학 표준을 준수하는지 여부를 평가하기 위해 체계적인 방법을 채택하기 시작했습니다.
의료기기 분야에서 인간 요소 및 사용성 엔지니어링에 대한 강조는 환자의 안전과 기기의 사용성에 대한 광범위한 헌신을 반영합니다. 이로 인해 제품수명주기 전반에 걸쳐(개발 후반부가 아닌) 제품수명주기 전반에 걸쳐 휴먼팩터 및 사용성 엔지니어링을 수행해야 할 필요성이 높아지고 있습니다. 이를 통해 장비 개발자는 사용자 만족을 실현하고 위험을 제거하기 위해 장비 개발 프로세스를 적시에 미세 조정할 수 있습니다. 혁신적이고 개인화된 의료기기에 대한 수요가 증가함에 따라 휴먼팩터 및 사용성 엔지니어링 서비스 아웃소싱은 가까운 시일 내에 증가할 것으로 예상됩니다.
현재 210개 이상의 기업이 다양한 의료기기에 대한 휴먼팩터 및 사용성 엔지니어링 서비스를 제공하는 데 필요한 전문 지식을 보유하고 있다고 주장하고 있습니다.
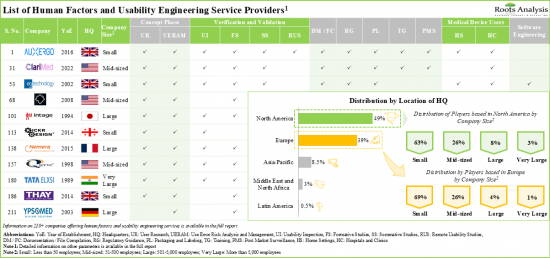
95% 이상의 서비스 프로바이더가 제품 설계 및 개발 관련 서비스를 제공하고 있으며, 그 중 대다수의 서비스 프로바이더가 진단 장비의 테스트를 지원하고 있습니다. 경쟁이 치열한 이 업계에서 우위를 점하기 위해 각 업체들은 인적 요소 엔지니어링 서비스 포트폴리오를 강화하기 위해 지속적으로 역량을 업그레이드하고 있습니다.
세계의 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장에 대해 조사했으며, 시장의 개요와 스텝별, 조사 방법 유형별, 테스트 대상 의료기기 클래스별, 지역별, 주요 기업별 동향 및 시장에 참여하는 기업의 개요 등을 제공하고 있습니다.
목차
제1장 서문
제2장 조사 방법
제3장 시장 역학
제4장 경제 및 기타 프로젝트 특유의 고려 사항
제5장 개요
제6장 서론
제7장 휴먼팩터와 유저빌리티 엔지니어링에 관한 규제 가이드라인
제8장 시장 구도
제9장 기업 경쟁력 분석
제10장 기업 개요 : 북미의 서비스 프로바이더
- 챕터 개요
- ClariMed
- Aptar Digital Health
- Bold Insight
- Greenlight Guru
- Key Winning Strategies
제11장 기업 개요 : 유럽의 서비스 프로바이더
- 챕터 개요
- Bayoomed
- AYES
- Comate
- Key Winning Strategies
제12장 기업 개요 : 아시아태평양의 서비스 프로바이더
- 챕터 개요
- Hydrix
- D+I
- Invetech
- Johari Digital
- Key Winning Strategies
제13장 합병과 인수
제14장 휴먼팩터와 유저빌리티 엔지니어링 프로세스에 관련된 비용의 영향
제15장 사례 연구 : 의료기기 리콜과 휴먼팩터 및 유저빌리티 엔지니어링의 역할
제16장 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장 : 진행중인 메가트렌드
제17장 시장 영향 분석
- 챕터 개요
- 시장 촉진요인
- 시장 억제요인
- 시장의 기회
- 시장이 해결해야 할 과제
- 결론
제18장 세계의 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장
제19장 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장, 스텝별
제20장 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장, 조사 방법 유형별
제21장 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장, 테스트 대상 의료기기 클래스별
제22장 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장, 지역별
제23장 휴먼팩터 및 유저빌리티 엔지니어링 서비스 시장, 주요 기업별
제24장 결론
제25장 이그제큐티브 인사이트
- 챕터 개요
- ClariMed
- Loring Human Factors
- Cambridge Design Partnership
- Ergosign
- DCA
- Thay Medical
제26장 부록 I : 표 형식 데이터
제27장 부록 II : 기업 및 단체 리스트
KSA 25.02.24HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: OVERVIEW
As per Roots Analysis, the global human factors and usability engineering services market is estimated to grow from USD 0.94 billion in the current year to USD 1.97 billion by 2035, at a CAGR of 6.9% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Steps Involved
- Summative Study
- Contextual Analysis
- Formative Study
- Known Use Error Analysis
- Use Risk Analysis
- Submission Preparation
- Design Process
- Task Analysis
Type of Research Method
- Evaluation Research
- Generative Research
Class of Medical Device Tested
- Class I Medical Devices
- Class II Medical Devices
- Class III Medical Devices
Key Geographical Regions
- North America (US, Canada)
- Europe (Germany, France, Italy, Switzerland, UK, Rest of
the Europe)
- Asia-Pacific (China, Japan, Australia, India, Rest of Asia-Pacific)
- Middle East and North Africa Latin America
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: GROWTH AND TRENDS
Over the years, human factors and usability engineering have become an integral part of the development of all classes of medical devices. As a result, the developers have actively started relying on the service providers for conducting human factors research for their devices (in development) in order to enhance device safety, efficacy and user experience. As medical devices become more complex, it is imperative to focus on the prototype and user interface design so as to minimize any potential risk associated with the use. Further, regulatory bodies (especially, the USFDA) are increasingly mandating the incorporation of human factors into the design and evaluation of medical devices. These bodies have started to employ a systematic method to assess manufacturers' adherence to usability engineering standards.
The emphasis on human factors and usability engineering in the medical device sector reflects a broader commitment to patient safety and device usability. This potentiates the need to conduct human factors and usability engineering on the devices throughout the product lifecycle (rather than conducting at later stages of development). This will support the device developers in timely facilitating the tweaks in the device development process so as to enable user satisfaction and eliminate risks. With the growing demand for innovative and personalized medical devices, the outsourcing of human factors and usability engineering services is anticipated to rise in the near future.
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: KEY INSIGHTS
The report delves into the current state of the human factors and usability engineering services market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Presently, over 210 companies claim to have the required expertise to offer human factors and usability engineering services for various medical devices; notably, most of the start-ups are based in Europe.
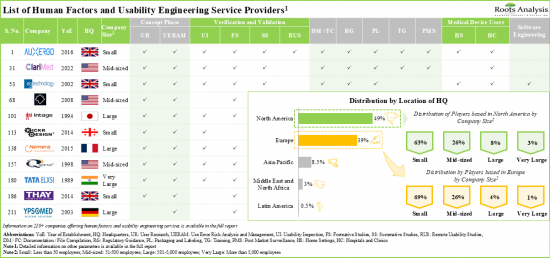
- More than 95% of the service providers offer product design and development related services; of these, majority of service providers support the testing of diagnostic devices.
- In order to gain an edge in this competitive industry, companies are continuously upgrading their capabilities to enhance their respective human factor engineering service portfolios.
- The rising interest in this domain is reflected by the wide array of mergers and acquisitions occurred in the recent past; in fact, close to 50% of the deals were inked in the last three years.
- Owing to the benefits offered by outsourcing human factors and usability engineering services, the market is anticipated to grow at an annualized rate of over 6.9% in the coming decade.
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: KEY SEGMENTS
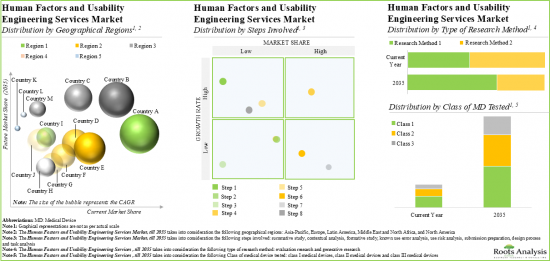
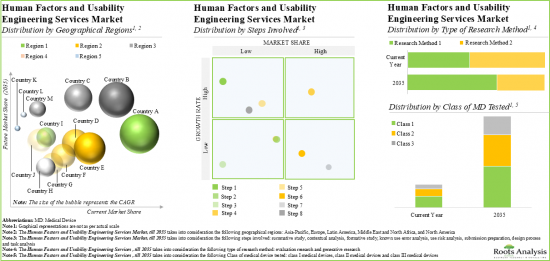
Currently, Summative Studies Account for the Largest Share of the Human Factors and Usability Engineering Services Market
Based on the steps involved, the market is segmented into summative study, contextual analysis, formative study, known use error analysis, use risk analysis, submission preparation, design process and task analysis. Currently, summative study holds the largest market share and is poised for the highest growth during the forecast period. This can be attributed to the fact that summative studies play a critical role in validating the safety and effectiveness of medical devices. Further, contextual analysis captures a significant share of the human factors and usability engineering services market. It is worth highlighting that contextual analysis offers various advantages, such as decreased chance of device recall, high return on investments (ROI) and expedition in the market launch of the medical device.
Evaluation Research is Likely to Dominate the Human Factors and Usability Engineering Services Market During the Forecast Period
Based on the type of research method, the market is segmented into evaluation research and generative research. At present, evaluation research segment occupies the highest market share of the human factors and usability engineering services market. This trend is unlikely to change in the near future.
Currently, Class III Medical Devices Testing Segment Occupies the Largest Share of the Human Factors and Usability Engineering Services Market
Based on the class of medical device tested, the market is segmented into class I, class II and class III. At present, class III medical device testing holds the maximum share of the human factors and usability engineering services market. This can be attributed to the fact that class III devices are considered high-risk devices and have the potential to cause serious illness or injury to the patients. Moreover, class II medical devices (including the IVD and injectors) require careful consideration of human factors engineering to ensure safe and effective use.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America. Majority share is expected to be captured by players based in North America and Europe. This can be attributed to the stringent regulatory guidelines for medical device approvals in this region. It is worth highlighting that, over the years, the market in the rest of the world is expected to grow at a higher CAGR.
Example Players in the Human Factors and Usability Engineering Services Market
- Aptar Digital Health
- AYES
- Bayoomed
- Bold Insight
- ClariMed
- Comate
- D+I
- Greenlight Guru
- Hydrix
- Invetech
- Johari Digital
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:
- Chief Executive Officer and Chair, ClariMed
- Managing Director and Human Factors Consultant, THAY Medical
- Head of Human Factors, Cambridge Design Partnership
- Head of Research (Human Factors and Interaction), DCA
- UX Director and Team Manager, Ergosign
HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the human factors and usability engineering services market, focusing on key market segments, including [A] steps involved, [B] type of research method, [C] class of medical device tested, and [D] geographical regions.
- Market Landscape: A comprehensive evaluation of human factors and usability engineering service providers, considering various parameters, such as [A] year of establishment, [B] company size (in terms of employee count), [C] location of headquarters, [D] type of service offered, [E] concept phase, [F] design and development, [G] verification and validation testing, [H] type of medical devices tested, [I] medical device users and [J] software engineering offered.
- Regulatory Landscape Analysis: A discussion on general regulatory guidelines established and issued by major regulatory bodies for medical device approval. In addition, it features a brief description of human factors and usability engineering process followed by regulatory bodies in the US and the EU, their comparison and a common approach that can be adopted to satisfy guidelines issued by authorities of the aforementioned regions.
- Company Competitiveness Analysis: A comprehensive competitive analysis of service providers engaged in the human factors and usability engineering market, examining factors such as [A] company strength and [B] portfolio strength.
- Company Profiles: In-depth profiles of key human factors and usability engineering service providers based in North America, Europe and Asia-Pacific, focusing on [A] company overviews, [B] service portfolio, [C] recent developments and [D] an informed future outlook.
- Mergers and Acquisitions: A comprehensive examination of the various mergers and acquisitions, focusing on multiple relevant parameters, including [A] year of agreement, [B] type of agreement and [C] geographical location of the companies.
- Case Study: The chapter discusses medical device recalls, focusing on the yearly trends in recalls and the potential underlying causes. It also highlights the top five medical device recalls and suggests strategies for incorporating human factors and usability engineering into medical devices to minimize errors and decrease the frequency of recalls.
- Mega Trends: A qualitative assessment of the various megatrends ongoing in the human factors and usability engineering market.
- Additional Insight: A detailed discussion on the cost implications across various steps of the human factors and usability engineering process, namely contextual inquiry, task analysis, design process, formative study, use risk analysis, known use error analysis, summative study, submission preparation, and the cost requirements across each of the aforementioned stages.
- Market Impact Analysis: The report analyzes various factors such as drivers, restraints, opportunities, and challenges affecting the market growth.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Forecast Methodology
- 3.2.1. Top-down Approach
- 3.2.2. Bottom-up Approach
- 3.2.3. Hybrid Approach
- 3.3. Market Assessment Framework
- 3.3.1. Total Addressable Market (TAM)
- 3.3.2. Serviceable Addressable Market (SAM)
- 3.3.3. Serviceable Obtainable Market (SOM)
- 3.3.4. Currently Acquired Market (CAM)
- 3.4. Forecasting Tools and Techniques
- 3.4.1. Qualitative Forecasting
- 3.4.2. Correlation
- 3.4.3. Regression
- 3.4.4. Extrapolation
- 3.4.5. Convergence
- 3.4.6. Sensitivity Analysis
- 3.4.7. Scenario Planning
- 3.4.8. Data Visualization
- 3.4.9. Time Series Analysis
- 3.4.10. Forecast Error Analysis
- 3.5. Key Considerations
- 3.5.1. Demographics
- 3.5.2. Government Regulations
- 3.5.3. Reimbursement Scenarios
- 3.5.4. Market Access
- 3.5.5. Supply Chain
- 3.5.6. Industry Consolidation
- 3.5.7. Pandemic / Unforeseen Disruptions Impact
- 3.6. Key Market Segmentation
- 3.7. Robust Quality Control
- 3.8. Limitations
4. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Future Estimates
- 4.2.2. Currency Coverage and Foreign Exchange Rate
- 4.2.2.1. Major Currencies Affecting the Market
- 4.2.2.2. Factors Affecting Currency Fluctuations and Foreign Exchange Rates
- 4.2.2.3. Impact of Foreign Exchange Rate Volatility on the Market
- 4.2.2.4. Strategies for Mitigating Foreign Exchange Risk
- 4.2.3. Trade Policies
- 4.2.3.1. Impact of Trade Barriers on the Market
- 4.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 4.2.4. Recession
- 4.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.1. Time Period
5. EXECUTIVE SUMMARY
- 5.1. Chapter Overview
- 5.2. Executive Summary: Market Landscape
- 5.3. Executive Summary: Mergers and Acquisitions
- 5.4. Executive Summary: Market Forecast and Opportunity Analysis
6. INTRODUCTION
- 6.1. Chapter Overview
- 6.2. Overview of Human Factors and Usability Engineering
- 6.3. Human Factors Influencing Medical Device Design
- 6.3.1. End Users
- 6.3.2. User Environment
- 6.3.3. Device-User Interface
- 6.4. Key Steps Involved in Human Factors and Usability Engineering
- 6.5. Outsourcing of Human Factors and Usability Engineering
- 6.6. Advantages of Outsourcing Human Factors and Usability Engineering
- 6.7. Risks and Challenges Associated with Outsourcing Human Factors and Usability Engineering
- 6.8. Key Considerations while Selecting a Human Factors and Usability Engineering Service Provider
- 6.9. Conclusion
7. REGULATORY GUIDELINES FOR HUMAN FACTORS AND USABILITY ENGINEERING
- 7.1. Chapter Overview
- 7.2. Key Regulatory Authorities for Human Factors and Usability Engineering
- 7.3. Regulatory Landscape in North America
- 7.3.1. The USFDA Recognized Standards on Human Factors
and Usability Engineering
- 7.3.2. The USFDA Guidance Documents Related to Human Factors and Usability Engineering
- 7.3.3. Human Factors and Usability Engineering Process by the USFDA
- 7.4. Regulatory Landscape in Europe
- 7.4.1. EU Recognized Standards for Human Factors and Usability Engineering
- 7.4.2. Human Factors and Usability Engineering Process by European Union
- 7.5. Comparison between the USFDA and EU Guidelines
- 7.5.1. Common Approach to Make the USFDA and EU Submissions
- 7.5.2. Human Factors and Usability Engineering Process for Other Devices
- 7.6. Recent Activity of Regulatory Bodies Involved
- 7.7. Concluding Remarks
8. MARKET LANDSCAPE
- 8.1. Chapter Overview
- 8.2. Human Factors and Usability Engineering Service Providers: Overall Market Landscape
- 8.2.1. Analysis by Year of Establishment
- 8.2.2. Analysis by Company Size
- 8.2.3. Analysis by Location of Headquarters
- 8.2.4. Analysis by Company Size and Location of Headquarters
- 8.2.5. Analysis by Type of Service Offered
- 8.2.5.1. Analysis by Concept Phase
- 8.2.5.2. Analysis by Design and Development
- 8.2.5.3. Analysis by Verification and Validation
- 8.2.6. Analysis by Type of Medical Device Tested
- 8.2.7. Analysis by End User of Medical Device Tested
- 8.2.8. Analysis by Software
Engineering Offered
9. COMPANY COMPETITIVENSS ANALYSIS
- 9.1. Chapter Overview
- 9.2. Assumptions and Key Parameters
- 9.3. Methodology
- 9.4. Company Competitiveness Analysis: Human Factors and Usability Engineering Service Providers
- 9.4.1. Human Factors and Usability Engineering Service Providers based in North America (Peer Group I)
- 9.4.2. Human Factors and Usability Engineering Service Providers based in Europe (Peer Group II)
- 9.4.3. Human Factors and Usability Engineering Service Providers based in Asia-Pacific, Middle East and North Africa, and Latin America (Peer Group III)
10. COMPANY PROFILES: SERVICE PROVIDERS IN NORTH AMERICA
- 10.1. Chapter Overview
- 10.2. ClariMed
- 10.2.1. Company Overview
- 10.2.2. Service Portfolio
- 10.2.3. Recent Developments and Future Outlook
- 10.3. Aptar Digital Health
- 10.3.1. Company Overview
- 10.3.2. Service Portfolio
- 10.3.3. Recent Developments and Future Outlook
- 10.4. Bold Insight
- 10.4.1. Company Overview
- 10.4.2. Service Portfolio
- 10.4.3. Recent Developments and Future Outlook
- 10.5. Greenlight Guru
- 10.5.1. Company Overview
- 10.5.2. Service Portfolio
- 10.5.3. Recent Developments and Future Outlook
- 10.6. Key Winning Strategies
11. COMPANY PROFILES: SERVICE PROVIDERS IN EUROPE
- 11.1. Chapter Overview
- 11.2. Bayoomed
- 11.2.1. Company Overview
- 11.2.2. Service Portfolio
- 11.2.3. Recent Developments and Future Outlook
- 11.3. AYES
- 11.3.1. Company Overview
- 11.3.2. Service Portfolio
- 11.3.3. Recent Developments and Future Outlook
- 11.4. Comate
- 11.4.1. Company Overview
- 11.4.2. Service Portfolio
- 11.4.3. Recent Developments and Future Outlook
- 11.5. Key Winning Strategies
12. COMPANY PROFILES: SERVICE PROVIDERS IN ASIA-PACIFIC
- 12.1. Chapter Overview
- 12.2. Hydrix
- 12.2.1. Company Overview
- 12.2.2. Service Portfolio
- 12.2.3. Recent Developments and Future Outlook
- 12.3. D+I
- 12.3.1. Company Overview
- 12.3.2. Service Portfolio
- 12.3.3. Recent Developments and Future Outlook
- 12.4. Invetech
- 12.4.1. Company Overview
- 12.4.2. Service Portfolio
- 12.4.3. Recent Developments and Future Outlook
- 12.5. Johari Digital
- 12.5.1. Company Overview
- 12.5.2. Service Portfolio
- 12.5.3. Recent Developments and Future Outlook
- 12.6. Key Winning Strategies
13. MERGERS AND ACQUISITIONS
- 13.1. Chapter Overview
- 13.2. Type of Mergers and Acquisitions
- 13.3. Mergers and Acquisitions: Human Factors and Usability Engineering Service Providers
- 13.3.1. Analysis by Year of Agreement
- 13.3.2. Analysis by Type of Agreement
- 13.3.3. Analysis by Geography
- 13.3.3.1. Intracontinental and Intercontinental Agreements
- 13.3.3.2. International and Local Agreements
- 13.4. Most Active Players: Analysis by Number of Agreements
14. COST IMPLICATIONS RELATED TO HUMAN FACTORS AND USABILITY ENGINEERING PROCESS
- 14.1. Chapter Overview
- 14.2. Key Steps involved in Human Factors and Usability Engineering Process
- 14.3. Cost Distribution across the Different Steps of Human Factors and Usability Engineering
- 14.3.1. Costs Associated with Contextual Inquiry
- 14.3.2. Costs Associated with Task Analysis
- 14.3.3. Costs Associated with Design Analysis
- 14.3.4. Costs Associated with Formative Study
- 14.3.5. Costs Associated with Use Risk Analysis
- 14.3.6. Costs Associated with Known Use Error Analysis
- 14.3.7. Costs Associated with Summative Study
- 14.3.8. Costs Associated with Submission Preparation
15. CASE STUDY: MEDICAL DEVICE RECALLS AND ROLE OF HUMAN FACTORS AND USABILITY ENGINEERING
- 15.1. Chapter Overview
- 15.2. Medical Device Recalls
- 15.3. Major Medical Device Recalls
- 15.3.1. Philips: Recall of Sense XL Torso Coil
- 15.3.2. Inspire Medical Systems: Recall of Inspire IV Implantable Pulse Generator (IPG) Model 3028
- 15.3.3. Hamilton Medical: Recall of HAMILTON-C6 Intensive Care Ventilator
- 15.3.4. Zoll Medical: Recall of ZOLL 731 Ventilator
- 15.3.5. Elekta Instrument: Recall of Disposable Biopsy Needle Kit
- 15.4. Medical Device Recalls: Role Of Human Factors and Usability Engineering
- 15.5. Conclusion
16. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET: ONGOING MEGATRENDS
- 16.1. Chapter Overview
- 16.2. Key Megatrends
17. MARKET IMPACT ANALYSIS
- 17.1. Chapter Overview
- 17.2. Market Drivers
- 17.3. Market Restraints
- 17.4. Market Opportunities
- 17.5. Market Challenges
- 17.6. Conclusion
18. GLOBAL HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET
- 18.1. Chapter Overview
- 18.2. Assumptions and Methodology
- 18.3. Global Human Factors and Usability Engineering Services Market, till 2035
- 18.3.1. Roots Analysis Perspective on Market Growth
- 18.3.2. Scenario Analysis
- 18.3.2.1. Conservative Scenario
- 18.3.2.2. Optimistic Scenario
- 18.4. Key Market Segmentations
19. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY STEPS INVOLVED
- 19.1. Chapter Overview
- 19.2. Assumptions and Methodology
- 19.3. Human Factors and Usability Engineering Services Market: Distribution by Steps Involved, Current Year and 2035
- 19.3.1. Human Factors and Usability Engineering Services Market for Summative Study, till 2035
- 19.3.2. Human Factors and Usability Engineering Services Market for Contextual Analysis, till 2035
- 19.3.3. Human Factors and Usability Engineering Services Market for Formative Study, till 2035
- 19.3.4. Human Factors and Usability Engineering Services Market for Known Use Error Analysis, till 2035
- 19.3.5. Human Factors and Usability Engineering Services Market for Use Risk Analysis, till 2035
- 19.3.6. Human Factors and Usability Engineering Services Market for Submission Preparation, till 2035
- 19.3.7. Human Factors and Usability Engineering Services Market for Design Analysis, till 2035
- 19.3.8. Human Factors and Usability Engineering Services Market for Task Analysis, till 2035
- 19.4. Penetration-Growth (P-G) Matrix
- 19.5. Data Triangulation and Validation
20. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY TYPE OF RESEARCH METHOD
- 20.1. Chapter Overview
- 20.2. Assumptions and Methodology
- 20.3. Human Factors and Usability Engineering Services Market: Distribution by Type of Research Method, Current Year and 2035
- 20.3.1. Human Factors and Usability Engineering Services Market for Evaluation Research, till 2035
- 20.3.2. Human Factors and Usability Engineering Services Market for Generative Research, till 2035
- 20.4. Penetration-Growth (P-G) Matrix
- 20.5. Data Triangulation and Validation
21. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY CLASS OF MEDICAL DEVICE TESTED
- 21.1. Chapter Overview
- 21.2. Assumptions and Methodology
- 21.3. Human Factors and Usability Engineering Services Market: Distribution by Class of Medical Device Tested, 2024 and 2035
- 21.3.1. Human Factors and Usability Engineering Services Market for Class I Medical Devices, till 2035
- 21.3.2. Human Factors and Usability Engineering Services Market for Class II Medical Devices, till 2035
- 21.3.3. Human Factors and Usability Engineering Services Market for Class III Medical Devices, till 2035
- 21.4. Penetration-Growth (P-G) Matrix
- 21.5. Data Triangulation and Validation
22. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY GEOGRAPHICAL REGIONS
- 22.1. Chapter Overview
- 22.2. Assumptions and Methodology
- 22.3. Human Factors and Usability Engineering Services Market: Distribution by Geographical Regions, Current Year and 2035
- 22.3.1. Human Factors and Usability Engineering Services Market in North America, till 2035
- 22.3.1.1. Human Factors and Usability Engineering Services Market in the US, till 2035
- 22.3.1.2. Human Factors and Usability Engineering Services Market in Canada, till 2035
- 22.3.2. Human Factors and Usability Engineering Services Market in Europe, till 2035
- 22.3.2.1. Human Factors and Usability Engineering Services Market in Germany, till 2035
- 22.3.2.2. Human Factors and Usability Engineering Services Market in France, till 2035
- 22.3.2.3. Human Factors and Usability Engineering Services Market in Italy, till 2035
- 22.3.2.4. Human Factors and Usability Engineering Services Market in the UK, till 2035
- 22.3.2.5. Human Factors and Usability Engineering Services Market in Switzerland, till 2035
- 22.3.2.6. Human Factors and Usability Engineering Services Market in Rest of Europe, till 2035
- 22.3.3. Human Factors and Usability Engineering Services Market in Asia-Pacific, till 2035
- 22.3.3.1. Human Factors and Usability Engineering Services Market in China, till 2035
- 22.3.3.2. Human Factors and Usability Engineering Services Market in Japan, till 2035
- 22.3.3.3. Human Factors and Usability Engineering Services Market in Australia, till 2035
- 22.3.3.4. Human Factors and Usability Engineering Services Market in India, till 2035
- 22.3.3.5. Human Factors and Usability Engineering Services Market in Rest of Asia-Pacific, till 2035
- 22.3.4. Human Factors and Usability Engineering Services Market in Middle East and North Africa, till 2035
- 22.3.5. Human Factors and Usability
- 22.3.1. Human Factors and Usability Engineering Services Market in North America, till 2035
Engineering Services Market in Latin America, till 2035
- 22.4. Market Movement Analysis
- 22.5. Penetration-Growth (P-G) Matrix
- 22.6. Data Triangulation and Validation
23. HUMAN FACTORS AND USABILITY ENGINEERING SERVICES MARKET, BY LEADING PLAYERS
- 23.1. Chapter Overview
- 23.2. Leading Industry Players
24. CONCLUSION
25. EXECUTIVE INSIGHTS
- 25.1. Chapter Overview
- 25.2. ClariMed
- 25.2.1. Company Snapshot
- 25.2.2. Interview Transcript: Chief Executive Officer and Chair
- 25.3. Loring Human Factors
- 25.3.1. Company Snapshot
- 25.3.2. Interview Transcript: Founder and Principal
- 25.4. Cambridge Design Partnership
- 25.4.1. Company Snapshot
- 25.4.2. Interview Transcript: Head of Human Factors
- 25.5. Ergosign
- 25.5.1. Company Snapshot
- 25.5.2. Interview Transcript: UX Director and Principal UX Designer
- 25.6. DCA
- 25.6.1. Company Snapshot
- 25.6.2. Interview Transcript: Head of Research (Human Factors and Interaction)
- 25.7. Thay Medical
- 25.7.1. Company Snapshot
- 25.7.2. Interview Transcript: Managing Director and Human Factors Consultant



















