
|
시장보고서
상품코드
1682717
나노입자 제제 시장 : 나노입자 유형별, 사업 규모별, 주요 지역별 - 업계 동향과 세계 예측(- 2035년)Nanoparticle Formulation Market by Type of Nanoparticle (Organic, Inorganic Nanoparticles and Carbon-based Nanoparticles), Scale of Operation and Key Geographical Regions : Industry Trends and Global Forecasts, Till 2035 |
||||||
세계 나노입자 제제 시장 규모는 2035년까지 예측 기간 동안 9.4%의 연평균 복합 성장률(CAGR)로 확대되어 현재 51억 달러에서 2035년까지 151억 달러로 성장할 것으로 예상됩니다.
수많은 질병에 대한 강력한 치료 가능성을 보여준 나노입자 기반 의약품은 단기간에 유망한 치료 옵션으로 떠올랐습니다. 실제로 전문가들은 현재 진행 중인 연구 속도를 고려할 때, 나노입자 분야의 놀라운 혁신(질병 진단 및 치료 특이성 개선)은 향후 몇년내에 제약업계에 혁명을 일으킬 가능성이 높다고 보고 있습니다. 또한, 매개성 질환의 검출 및 제어, 투석, 분자 이미징을 위한 나노기술에 초점을 맞춘 최근의 노력은 이러한 나노입자에 대한 수요를 더욱 촉진하고 있습니다. 또한, 나노입자는 변경 가능한 특성(크기, 모양, 표면 특성, 전하 등)으로 인해 최적의 전달을 달성하고 환자 체내의 생물학적 장벽(전신, 미세환경, 세포)의 제한을 극복할 수 있도록 설계할 수 있습니다. 그러나 나노입자의 특성과 생물학적 시스템 간의 복잡한 상호작용은 이러한 나노 스케일 물질의 안전성과 유효성을 보장하기 위해 신중한 검토가 필요하며, CRO(임상시험수탁기관) 및 CDO/CDMO(의약품 개발 및 제조 수탁기관)와 같은 다양한 이해관계자 는 연구자 및 의약품 개발자들이 설계, 개발, 제조와 관련된 복잡한 문제들을 잘 헤쳐나갈 수 있도록 지원하는 데 있어 매우 중요한 역할을 할 것으로 예상됩니다. 위탁 서비스 제공업체는 이 분야의 다각적인 전문성을 활용하여 나노입자 개발 프로세스를 간소화하고, 비용을 절감하며, 유망한 나노입자의 실험실 규모에서 임상 규모로의 전환을 촉진하는 데 도움을 줄 수 있습니다. 기술 발전과 나노입자 기반 의약품에 대한 수요 증가에 힘입어 나노입자 제제 서비스 시장은 향후 상당한 성장을 이룰 것으로 예상됩니다.
현재, 나노입자 기반 의약품의 개발 및 제조를 강화하기 위해 전 세계 100개 이상의 나노입자 제제 기술이 제공되고 있습니다. 기술의 약 80%는 유기 나노입자 제형화를 위해 설계되었으며, 그 중 약 50%는 주로 종양 질환을 대상으로 하는 주사 경로를 통한 약물 전달을 목표로 하고 있습니다. 현재 80개 이상의 서비스 제공업체가 다양한 규모의 나노입자 제제 서비스를 제공합니다.
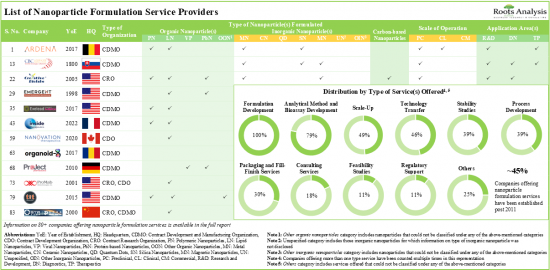
시장 환경은 광범위한 응용 분야에 대한 나노입자 기반 의약품을 개발/제조하는 데 필요한 전문 지식을 갖춘 기존 기업과 신규 진출기업이 모두 존재한다는 특징이 있습니다. 나노입자 기반 제제에 대한 수요가 증가함에 따라 서비스 제공업체들은 기존 역량을 지속적으로 확장하여 서비스 포트폴리오를 강화하고 있습니다. 최근 몇 년동안 파트너십 활동의 꾸준한 성장이 관찰되고 있습니다. R&D 계약은 미국에 본사를 둔 기업들이 채택하는 가장 두드러진 유형의 파트너십 모델입니다. 지적재산권 보호를 위해 나노입자 제형 및 개발과 관련된 1,700개 이상의 특허가 다양한 기관에 출원되었으며, 다양한 기관에 특허가 부여되었습니다. 나노입자 제제 서비스 시장은 상업적 규모의 운영에서 발생하는 수익에 힘입어 향후 10년간 연평균 9%의 성장률을 보일 것으로 예상됩니다.
세계의 나노입자 제제 시장에 대해 조사했으며, 시장 개요와 함께 나노입자 유형별/사업 규모별/주요 지역별 동향, 시장 진출 기업 프로파일 등의 정보를 전해드립니다.
목차
제1장 서문
제2장 주요 요약
제3장 서론
제4장 기술 상황
제5장 서비스 제공업체 상황
제6장 기술 경쟁력 분석
제7장 기업 경쟁력 분석
제8장 기업 개요
- 본 장의 개요
- Ascension Sciences
- DIANT Pharma
- ExonanoRNA
- Nanoform
- NanoVation Therapeutics
- NanoVelos
- NTT Biopharma
- Organoid-X BioTech
- Vaxinano
제9장 파트너십 및 협업
제10장 특허 분석
제11장 나노입자 평가 프레임워크
제12장 시장 예측과 기회 분석
제13장 사례 연구 : 기술 라이선싱 계약
제14장 결론
제15장 주요 인사이트
제16장 부록 1 : 표 형식 데이터
제17장 부록 2 : 기업 및 단체 리스트
LSH 25.03.27NANOPARTICLE FORMULATION MARKET: OVERVIEW
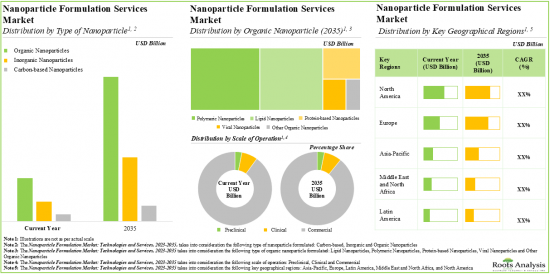
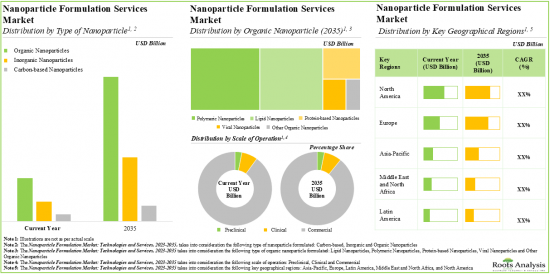
As per Roots Analysis, the global nanoparticle formulation market is estimated to grow from USD 5.1 billion in the current year to USD 15.1 billion by 2035, at a CAGR of 9.4% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Nanoparticle Formulated
- Organic Nanoparticles
- Inorganic Nanoparticles
- Carbon-based Nanoparticles
Type of Organic Nanoparticle Formulated
- Polymeric Nanoparticles
- Lipid Nanoparticles
- Viral Nanoparticles
- Protein-based Nanoparticles
- Other Organic Nanoparticles
Scale of Operation
- Preclinical
- Clinical
- Commercial
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
- Latin America
NANOPARTICLE FORMULATION MARKET: GROWTH AND TRENDS
Having demonstrated a strong therapeutic potential across a myriad of diseases, nanoparticle-based drugs have emerged as a promising treatment alternative within a short span. In fact, given the current ongoing pace of research, experts believe that remarkable innovations in the field of nanoparticles (in terms of improvement in disease diagnosis and treatment specificity) are likely to revolutionize the pharma industry in the coming years. Furthermore, the recent initiatives focused on nanotechnology for the detection and control of vector-borne diseases, dialysis, and molecular imaging, have further fueled the demand for these nanoparticles. In addition, the nanoparticles can be engineered to achieve optimal delivery and overcome the limitations of biological barriers (systemic, microenvironmental and cellular) in the patient's body owing to their modifiable properties (such as size, shape, surface properties and charge). However, the complex interplay between nanoparticle properties and biological systems requires careful consideration to ensure the safety and efficacy of these nanoscale materials. Various stakeholders, such as contract research organizations, (CROs), and contract development and manufacturing organizations (CDOs / CDMOs) are likely to play a pivotal role in supporting researchers and drug developers to navigate the complexities associated with its design, development and manufacturing. By leveraging the multifaceted expertise in the field, contract service providers can help streamline the nanoparticle development process, reduce costs, and accelerate the translation of promising nanoparticles from lab scale to clinical scale. Driven by technological advancements and the rising demand for nanoparticle-based drugs, the nanoparticle formulation services market is anticipated to witness notable growth in the foreseen future.
NANOPARTICLE FORMULATION MARKET: KEY INSIGHTS
The report delves into the current state of the nanoparticle formulation market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Presently, more than 100 nanoparticle formulation technologies are being offered by companies across the globe in order to augment the development and manufacturing of nanoparticle-based drugs.
- Close to 80% of technologies are designed for the formulation of organic nanoparticles; of these, nearly 50% are intended for drug delivery via injectable route, primarily targeting oncological disorders.
- The current service providers landscape has over 80 companies offering a wide range of nanoparticle formulation services at various scales of operation.
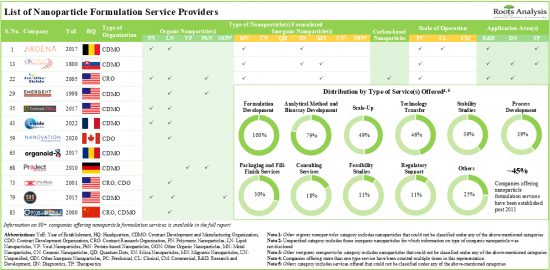
- The market landscape features the presence of both well-established players and new entrants with the requisite expertise to develop / manufacture nanoparticle-based drugs for an extensive range of application areas.
- Owing to the rise in demand for nanoparticle-based formulations, service providers are continuously expanding their existing capabilities to enhance their respective service portfolios.
- A steady growth in the partnership activity has been observed in recent years; R&D agreements have been the most prominent type of partnership model adopted by players based in the US.
- More than 1,700 patents related to nanoparticle formulation and development have been filed by / granted to various organizations in order to protect intellectual property.
- The nanoparticle formulation services market is likely to grow at a CAGR of ~9% over the next decade, driven by revenues generated from commercial scale operation.
NANOPARTICLE FORMULATION MARKET: KEY SEGMENTS
Organic Nanoparticles are Likely to Dominate the Nanoparticle Formulation Market During the Forecast Period
Based on the type of nanoparticle, the market is segmented into organic nanoparticles, inorganic nanoparticles and carbon-based nanoparticles. At present, organic nanoparticles hold the maximum share of the nanoparticle formulation market. This trend is unlikely to change in the near future.
Protein-based Nanoparticles Segment is the Fastest Growing Segment of the Nanoparticle Formulation Market During the Forecast Period
Based on the type of organic nanoparticle, the market is segmented into polymeric nanoparticles, lipid nanoparticles, viral nanoparticles, protein-based nanoparticles, and other organic nanoparticles. It is worth highlighting that, currently, lipid nanoparticles hold a larger share of the nanoparticle formulation market. However, the nanoparticle formulation market for protein-based nanoparticles is likely to grow at a higher CAGR in the coming decade.
Commercial Operations Occupy the Largest Share of the Nanoparticle Formulation Market
Based on scales of operation, the market is segmented into preclinical, clinical and commercial scales. At present, commercial operations capture the maximum share of the nanoparticle formulation market. It is worth highlighting that the nanoparticle formulation market at the clinical scale is likely to grow at a higher CAGR in the near future.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the world. The majority share is expected to be captured by service providers based in North America and Europe. It is worth highlighting that, over the years, the market in Asia-Pacific is expected to grow at a higher CAGR.
Example Players in the Nanoparticle Formulation Market
- Ascension Sciences
- DIANT Pharma
- ExonanoRNA
- Nanoform
- NanoVation Therapeutics
- NanoVelos
- NTT Biopharma
- Organoid-X BioTech
- Vaxinano
NANOPARTICLE FORMULATION MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the nanoparticle formulation market, focusing on key market segments, including [A] type of nanoparticle formulated, [B] type of organic nanoparticle formulated, [C] scale of operation and [D] key geographical regions.
- Market Landscape 1: A comprehensive evaluation of nanoparticle formulation technologies, considering various parameters, such as [A] type of nanoparticle(s) formulated, [B] type of molecule(s) delivered, [C] therapeutic area(s), [D] compatible dosage form(s) and [E] route(s) of administration. Further, the chapter provides information on various technology developers, along with analysis based on multiple parameters, such as [F] year of establishment, [G] company size, [H] location of headquarters and [I] most active players (in terms of number of technologies developed).
- Market Landscape 2: A comprehensive evaluation of nanoparticle formulation service providers, considering various parameters, such as [A] year of establishment, [B] company size (in terms of number of employees), [C] location of headquarters, [D] location of facilities, [E] type of service provider(s), [F] type of nanoparticle(s) formulated, [G] type of service(s) offered, [H] scale of operation and [I] application area(s).
- Technology Competitiveness: A comprehensive competitive analysis of nanoparticle formulation technologies, examining factors, such as [A] developer power, [B] technology strength and [C] technology applicability.
- Company Competitiveness: A comprehensive competitive analysis of nanoparticle formulation service providers, examining factors, such as [A] company strength and [B] service strength.
- Company Profiles: In-depth profiles of key industry players offering technologies and services for nanoparticle formulation across North America, Europe and Asia-Pacific, focusing on [A] company overviews, [B] technology portfolio, [C] service portfolio, [D] recent developments and [E] an informed future outlook.
- Partnerships and Collaborations: An analysis of partnerships established in this sector, since 2018, covering technology licensing agreements, research and development agreements, product development agreements, manufacturing agreements, mergers and acquisitions, technology integration agreements and other relevant agreements.
- Patent Analysis: Detailed analysis of various patents filed / granted related to nanoparticle formulation based on [A] publication year, [B] geographical region, [C] CPC symbols, [D] leading players (in terms of number of patents filled / granted) and [E] type of organization. It also includes a patent benchmarking analysis and a detailed valuation analysis.
- Nanoparticle Evaluation Framework: An insightful framework evaluating types of nanoparticles based on various parameters, such as [A] number of technologies, [B] nanoparticle efficacy, [C] number of clinical trials evaluating nanoparticle-based drugs, [D] extent of innovation, [E] trends in research activity and [F] current global competition. It also provides a value addition matrix for respective types of nanoparticles currently adopted by stakeholders.
- Case Study: A case study of the recent technology licensing agreements, along with information on deal amounts.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Research Methodology
- 1.5. Frequently Asked Questions
- 1.6. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to Nanoparticles
- 3.3. Classification of Nanoparticles
- 3.3.1. Organic Nanoparticles
- 3.3.2. Inorganic Nanoparticles
- 3.3.3. Carbon-based Nanoparticles
- 3.4. Methods of Nanoparticle Formulation
- 3.5. Applications of Nanoparticle-based Systems
- 3.6. Challenges associated with Nanoparticle Formulation
- 3.7. Need for Outsourcing Nanoparticle Formulation
- 3.8 Concluding Remarks
4. TECHNOLOGY LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Nanoparticle Formulation: Technology Landscape
- 4.2.1. Analysis by Type of Nanoparticle(s) Formulated
- 4.2.2. Analysis by Type of Organic Nanoparticle(s) Formulated
- 4.2.3. Analysis by Type of Inorganic Nanoparticle(s) Formulated
- 4.2.4. Analysis by Type of Molecule(s) Delivered
- 4.2.5. Analysis by Therapeutic Area(s)
- 4.2.6. Analysis by Compatible Dosage Form(s)
- 4.2.7. Analysis by Route(s) of Administration
- 4.3. Nanoparticle Formulation: Technology Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters
- 4.3.4. Analysis by Company Size and Location of Headquarters
- 4.3.5. Most Active Players: Analysis by Number of Technologies
5. SERVICE PROVIDERS LANDSCAPE
- 5.1. Chapter Overview
- 5.2. Nanoparticle Formulation: Service Providers Landscape
- 5.2.1. Analysis by Year of Establishment
- 5.2.2. Analysis by Company Size
- 5.2.3. Analysis by Location of Headquarters
- 5.2.4. Analysis by Company Size and Location of Headquarters
- 5.2.5. Analysis by Location of Facilities
- 5.2.6. Analysis by Type of Service Provider(s)
- 5.2.7. Analysis by Type of Nanoparticle(s) Formulated
- 5.2.7.1. Analysis by Type of Organic Nanoparticle(s) Formulated
- 5.2.7.2. Analysis by Type of Inorganic Nanoparticle(s) Formulated
- 5.2.8. Analysis by Type of Service Provider(s) and Type of Nanoparticle(s) Formulated
- 5.2.9. Analysis by Service(s) Offered
- 5.2.10. Analysis by Company Size and Service(s) Offered
- 5.2.11. Analysis by Scale of Operation
- 5.2.12. Analysis by Application Area(s)
6. TECHNOLOGY COMPETITIVENESS ANALYSIS
- 6.1. Chapter Overview
- 6.2. Assumptions / Key Parameters
- 6.3. Methodology
- 6.4. Technology Competitiveness Analysis
- 6.4.1. Nanoparticle Formulation Technologies Offered by Players based in North America
- 6.4.1.1. Nanoparticle Formulation Technologies Offered by Small Players based in North America
- 6.4.1.2. Nanoparticle Formulation Technologies Offered by Mid-sized and Large Players based in North America
- 6.4.2. Nanoparticle Formulation Technologies Offered by Players based in Europe
- 6.4.3. Nanoparticle Formulation Technologies Offered by Players based in Asia-Pacific, Middle East and North Africa, and Rest of the World
- 6.4.1. Nanoparticle Formulation Technologies Offered by Players based in North America
7. COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Assumptions / Key Parameters
- 7.3. Methodology
- 7.4. Company Competitiveness Analysis
- 7.4.1. Nanoparticle Formulation Service Providers based in North America
- 7.4.2. Nanoparticle Formulation Service Providers based in Europe
- 7.4.3. Nanoparticle Formulation Service Providers based in Asia-Pacific, Middle East and North Africa
8. COMPANY PROFILES
- 8.1. Chapter Overview
- 8.2. Ascension Sciences
- 8.2.1. Company Overview
- 8.2.2. Technology Portfolio
- 8.2.3. Service Portfolio
- 8.2.4. Recent Developments and Future Outlook
- 8.3. DIANT Pharma
- 8.3.1. Company Overview
- 8.3.2. Technology Portfolio
- 8.3.3. Service Portfolio
- 8.3.4. Recent Developments and Future Outlook
- 8.4. ExonanoRNA
- 8.4.1. Company Overview
- 8.4.2. Technology Portfolio
- 8.4.3. Service Portfolio
- 8.4.4. Recent Developments and Future Outlook
- 8.5. Nanoform
- 8.5.1. Company Overview
- 8.5.2. Technology Portfolio
- 8.5.3. Service Portfolio
- 8.5.4. Recent Developments and Future Outlook
- 8.6. NanoVation Therapeutics
- 8.6.1. Company Overview
- 8.6.2. Technology Portfolio
- 8.6.3. Service Portfolio
- 8.6.4. Recent Developments and Future Outlook
- 8.7. NanoVelos
- 8.7.1. Company Overview
- 8.7.2. Technology Portfolio
- 8.7.3. Service Portfolio
- 8.7.4. Recent Developments and Future Outlook
- 8.8. NTT Biopharma
- 8.8.1. Company Overview
- 8.8.2. Technology Portfolio
- 8.8.3. Service Portfolio
- 8.8.4. Recent Developments and Future Outlook
- 8.9. Organoid-X BioTech
- 8.9.1. Company Overview
- 8.9.2. Technology Portfolio
- 8.9.3. Service Portfolio
- 8.9.4. Recent Developments and Future Outlook
- 8.10. Vaxinano
- 8.10.1. Company Overview
- 8.10.2. Technology Portfolio
- 8.10.3. Service Portfolio
- 8.10.4. Recent Developments and Future Outlook
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Nanoparticle Formulation Technologies and Services: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Year and Type of Partnership
- 9.3.4. Analysis by Type of Partner
- 9.3.5. Analysis by Location of Headquarters of Partner
- 9.3.6. Analysis by Type of Partnership and Location of Headquarters of Partner
- 9.3.7. Most Active Players: Analysis by Number of Partnerships
- 9.3.8. Analysis by Geography
- 9.3.8.1. Intercontinental and Intracontinental Deals
- 9.3.8.2. Local and International Deals
10. PATENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Nanoparticle Formulation Domain: Patent Analysis
- 10.3.1. Analysis by Publication Year
- 10.3.2. Analysis by Type of Patent and Publication Year
- 10.3.3. Analysis by Geography
- 10.3.4. Analysis by CPC Symbols
- 10.3.5. Analysis by Type of Organization
- 10.3.6. Leading Industry Players: Analysis by Number of Patents
- 10.4. Patent Benchmarking Analysis
- 10.4.1. Analysis by Patent Characteristics
- 10.5. Patent Valuation Analysis
11. NANOPARTCLE EVALUATION FRAMEWORK
- 11.1. Chapter Overview
- 11.2. Key Assumptions and Methodology
- 11.3. Organic Nanoparticles
- 11.3.1. Number of Clinical Trials
- 11.3.2 Extent of Innovation
- 11.3.3. Trends in Research Activity
- 11.3.4. Current Global Competition
- 11.3.5. Nanoparticle Evaluation Framework: Organic Nanoparticles
- 11.4. Inorganic Nanoparticles
- 11.4.1. Number of Clinical Trials
- 11.4.2 Extent of Innovation
- 11.4.3. Trends in Research Activity
- 11.4.4. Current Global Competition
- 11.4.5. Nanoparticle Evaluation Framework: Inorganic Nanoparticles
- 11.5. Carbon-based Nanoparticles
- 11.5.1. Number of Clinical Trials
- 11.5.2 Extent of Innovation
- 11.5.3. Trends in Research Activity
- 11.5.4. Current Global Competition
- 11.5.5. Nanoparticle Evaluation Framework: Carbon-based Nanoparticles
- 11.5.6. Nanoparticle Evaluation Framework: Concluding Remarks
12. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 12.1. Chapter Overview
- 12.2. Key Assumptions and Forecast Methodology
- 12.3. Global Nanoparticle Formulation Services Market, till 2035
- 12.4. Nanoparticle Formulation Services Market: Analysis by Type of Nanoparticle Formulated
- 12.4.1. Nanoparticle Formulation Services Market: Analysis by Type of Organic Nanoparticle Formulated
- 12.4.1.1. Nanoparticle Formulation Services Market for Polymeric Nanoparticles, till 2035
- 12.4.1.2. Nanoparticle Formulation Services Market for Lipid Nanoparticles, till 2035
- 12.4.1.3. Nanoparticle Formulation Services Market for Viral Nanoparticles, till 2035
- 12.4.1.4. Nanoparticle Formulation Services Market for Protein-based Nanoparticles, till 2035
- 12.4.1.5. Nanoparticle Formulation Services Market for Other Organic Nanoparticles, till 2035
- 12.4.2. Nanoparticle Formulation Services Market: Analysis by Inorganic Nanoparticle Formulated
- 12.4.3. Nanoparticle Formulation Services Market: Analysis by Carbon-based Nanoparticle Formulated
- 12.4.1. Nanoparticle Formulation Services Market: Analysis by Type of Organic Nanoparticle Formulated
- 12.5. Nanoparticle Formulation Services Market: Analysis by Scale of Operation
- 12.5.1. Nanoparticle Formulation Services Market for Preclinical Operations, till 2035
- 12.5.2. Nanoparticle Formulation Services Market for Clinical Operations, till 2035
- 12.5.3. Nanoparticle Formulation Services Market for Commercial Operations, till 2035
- 12.6. Nanoparticle Formulation Services Market: Analysis by Key Geographical Regions
- 12.6.1. Nanoparticle Formulation Services Market in North America, till 2035
- 12.6.2. Nanoparticle Formulation Services Market in Europe, till 2035
- 12.6.3. Nanoparticle Formulation Services Market in Asia-Pacific, till 2035
- 12.6.4. Nanoparticle Formulation Services Market in Middle East and North Africa, till 2035
- 12.6.5. Nanoparticle Formulation Services Market in Latin America, till 2035
13. CASE STUDY: TECHNOLOGY LICENSING DEALS
- 13.1. Chapter Overview
- 13.2. Recent Technology Licensing Deals
- 13.3. Concluding Remarks



















