
|
시장보고서
상품코드
1723660
RSV 백신 시장 : 백신 유형별, 투여 경로별, 대상 환자층별, 유통 채널별, 주요 지역별, 주요 기업별 업계 동향 및 예측(-2040년)RSV Vaccine Market by Type of Vaccine, Route of Administration, Target Patient Population, Distribution Channel, Key Geographical Regions, and Leading Players: Industry Trends and Global Forecasts, till 2040 |
||||||
RSV 백신 시장
세계의 RSV 백신 시장 규모는 올해 11억 4,900만 달러에 달했습니다. 이 시장은 2040년에는 7억 3,100만 달러가 될 것으로 예측되고 있습니다.
RSV 백신 시장 기회는 다음 부문에 분포되어 있습니다.
백신 유형
- 서브유닛 및 바이러스 유사 입자(VLP) 백신
- 생백신
- mRNA 백신
투여 경로
- 근육내
- 기타
대상 환자층
- 고령자
- 영유아 및 소아
- 임산부
유통 채널
- 약국 및 약전문점
- 정부 및 기관 납품업체
- 기타
주요 지역
- 북미
- 유럽
RSV 백신 시장 : 성장 및 동향
호흡기 합포체 바이러스(RSV)는 유아, 고령자, 면역 결핍자에게 하기도 감염을 만연시키는 일반적인 바이러스입니다. 이 바이러스는 감염력이 강한 호흡기 병원체로 인식되고 있으며, 전 세계적으로 위중한 질환이나 사망의 원인이 되고 있습니다. WHO에 따르면 전 세계에서 5세 미만 소아에게 매년 약 3,300만 건의 RSV 감염증이 새롭게 발생하고 있습니다. 이는 RSV 감염증에 대한 효과적인 치료법이 없는 것에 기인하고 있습니다.
현재 RSV 감염의 치료에는 시판약, 점비약, 가습기 등 몇 가지 예방법이 사용되고 있습니다. 그러나 이들은 대증요법에 불과하기 때문에 안전하고 효과적인 표적 치료를 환자에게 제공하는 백신이 유망한 선택지가 되고 있습니다. 이들 백신은 기관지염이나 폐렴 등 호흡기 질환뿐만 아니라 RSV 감염증과 관련된 입원율, 합병증, 사망률을 최소화할 가능성을 내포하고 있습니다.

RSV 백신 개발을 위한 mRNA와 나노입자 기술의 사용 등 기술의 진보에 힘입어 이들 백신은 RSV 감염에 대하여 장기간의 면역과 효과적인 치료를 제공합니다. 더불어 RSV 치료를 위한 연구개발 노력 및 임상시험 건수 증가로 RSV 백신 수요는 증가할 것으로 예상되며, 시장은 향후 몇 년간 건전한 성장을 이룰 것으로 보입니다.
RSV 백신 시장 : 주요 인사이트
이 보고서는 RSV 백신 시장의 현재 상태를 조사하고 업계 내 잠재적 성장 기회를 확인합니다. 주요 조사결과는 다음과 같습니다. :
- 현재 70유형 이상의 RSV 백신이 다양한 기술을 이용하여 개발되고 있으며, 특히 이들 백신의 60% 이상이 다양한 임상시험 단계에서 검토되고 있습니다.
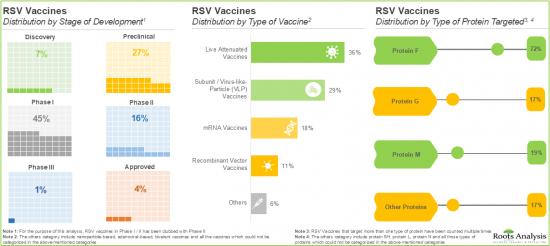
- RSV 백신에 대한 수요 증가는 백신 파이프라인의 현저한 증가를 가져오고, 현재 약 65종의 백신이 전임상 및 임상 개발 단계에 있습니다.
- RSV 백신을 평가하는 임상시험의 85% 이상은 업계 기업이 스폰서가 되고 있습니다.

- 2020년 이후, RSV 백신 영역에서는 330건 이상의 특허가 출원 및 부여되어 있으며, 특허의 대부분은 북미에서 출원되었습니다(50% 이상).
- 호흡기 합포체 바이러스 감염의 유행이 확대되고 있는 것과 기술 진보의 페이스가 증가하고 있는 것이, RSV 백신 시장을 견인하고 있어, 당분간은 안정된 성장이 전망됩니다.
- RSV 백신 시장의 전반적인 기회는 다양한 주요 지역, 백신 유형, 투여 경로, 대상 환자 집단, 유통 채널로 분산될 것으로 예측됩니다.
RSV 백신 시장 : 주요 부문
백신 유형에 의해 세계의 RSV 백신 시장은 서브유닛 및 바이러스 유사 입자(VLP) 백신, 약독화 생백신과 mRNA 백신으로 구분됩니다. 현재 RSV 백신 시장의 대부분의 점유율을 서브유닛 및 바이러스 유사입자(VLP) 백신이 차지하고 있습니다. 이는 이들 백신이 모바이러스와 형태적으로 유사하기 때문에 강한 면역원성 반응이 생기기 때문입니다.
세계의 RSV 백신 시장은 투여 경로에 따라 근육내 투여와 기타 경로로 나뉩니다. 현재 근육내 경로로 투여되는 백신이 시장 전체를 지배할 가능성이 높습니다. 이는 효율적인 흡수를 보장하는 높은 바이오 어베이러빌리티와 부작용 경감에 의해 환자에게 보다 안전하고 효과적인 선택지가 되는 것에 기인하고 있습니다.
세계의 RSV 백신 시장은 대상으로 하는 환자층에 의해 고령자, 유아 및 소아, 임신 중의 환자로 구분됩니다. 현재의 RSV 백신 시장은 노인 부문이 지배적일 것입니다. 이는 고령자가 호흡기 질환, 특히 RSV 시즌 중에 매우 이환하기 쉬운 것에 기인하고 있습니다.
세계의 RSV 백신 시장은 유통 채널별로 약국 및 약전문점, 정부 및 기관 납품업자, 기타로 구분됩니다. 주목할 점은 약국 및 약전문점 부문이 예측 기간 동안 RSV 백신 시장을 독점할 가능성이 높다는 점입니다. 이는 편의성이 높고 널리 이용하기 쉬운 데다 백신 접종률 상승에 기여하고 있는 약사에 의한 백신 투여 인가가 높아지고 있기 때문입니다.
주요 지역별로 보면 시장은 북미와 유럽으로 구분됩니다. 현재 북미가 최대 시장 점유율을 획득하고 있는데, 이는 이 지역에서의 호흡기 감염증 증가, 견고한 규제 프레임워크, 선진적인 헬스케어 인프라에 기인하고 있습니다.
이 보고서에서 답변을 얻을 수 있는 주요 질문
- 현재, 몇사가 이 시장에 참가하고 있는지
- 이 시장의 주요 기업
- 이 시장의 진화에 영향을 줄 것 같은 요인
- 현재 및 미래 시장 규모
- 이 시장의 CAGR
- 현재 및 미래 시장 기회는 주요 시장 부문에 어떻게 분배될 것인가
- 시장에서의 특허 출원 동향
이 보고서를 구입하는 이유
- 이 보고서는 종합적인 시장 분석을 제공하며 시장 전체와 특정 하위 부문에 대한 자세한 수익 예측을 제공합니다. 이 정보는 이미 시장을 선도하고 있는 기업에 있어서도, 신규 참가 기업에 있어서도 귀중한 것입니다.
- 이해관계자는 시장 내 경쟁 역학을 보다 깊이 이해하기 위해 보고서를 활용할 수 있습니다. 경쟁 상황을 분석함으로써 기업은 시장에서의 포지셔닝을 최적화하고 효과적인 시장 진입 전략을 개발하기 위해 정보에 입각한 의사결정을 할 수 있습니다.
- 이 보고서는 주요 촉진요인 및 시장 성장 억제요인과 과제 등 시장의 종합적인 개요를 이해관계자에게 제공합니다. 이 정보는 이해관계자가 시장 동향을 항상 파악하고 성장 전망을 활용하기 위한 데이터 주도의 의사 결정을 하기 위한 힘이 됩니다.
기타 혜택
- 보고서의 모든 분석 모듈의 무료 엑셀 데이터 팩
- 10% 무료 컨텐츠 커스터마이즈
- 조사팀에 의한 상세 보고서의 워크스루 세션
- 보고서가 6-12개월 이상 전인 경우, 무료 갱신 보고서
본 보고서에서는 세계의 RSV 백신 시장에 대해 조사했으며, 시장 개요와 함께, 백신 유형별, 투여 경로별, 대상 환자층별, 유통 채널별, 주요 지역별, 주요 기업별 동향, 시장 진출 기업 프로파일 등의 정보를 제공합니다.
목차
섹션 I : 보고서 개요
제1장 배경
제2장 조사 방법
제3장 시장 역학
제4장 거시경제지표
섹션 II : 질적 인사이트
제5장 주요 요약
제6장 서문
- RSV 바이러스 백신의 소개
- RSV 구조 및 메커니즘
- RSV 바이러스 백신과 관련된 주요 역사적 사건
- RSV 바이러스 백신 유형
- RSV 바이러스 백신 접종의 장점
- RSV 바이러스 백신 개발에 수반하는 과제
- 장래의 전망
섹션 III : 시장 개요
제7장 시장 상황
- RSV 바이러스 백신 : 시장 상황
제8장 제품 경쟁력 분석
- 전제 및 주요 파라미터
- 조사 방법
- RSV 바이러스 백신 : 제품 경쟁력 분석
섹션 IV : 기업 프로파일
제9장 기업 프로파일 : 북미의 RSV 바이러스 백신 개발 기업
- Icosavax(A Company of AstraZeneca)
- Moderna
- Pfizer
제10장 기업 프로파일 : 유럽의 RSV 백신 개발 기업
- GlaxoSmithKline
- Sanofi
제11장 기업 프로파일 : 아시아태평양의 RSV 백신 개발 기업
- Beijing Advaccine Biotechnology
제5장 시장 동향
제12장 임상시험 분석
- 범위 및 조사 방법
- RSV 바이러스 백신 : 임상시험 분석
제13장 특허 분석
제14장 FDA 승인 전략
섹션 VI : 시장 기회 분석
제15장 시장 영향 분석 : 촉진요인, 억제요인, 기회, 과제
- 시장 성장 촉진요인
- 시장 성장 억제요인
- 시장 기회
- 시장의 과제
제16장 세계의 RSV 백신 시장
제17장 RSV 바이러스 백신 시장 : 백신 유형별
제18장 RSV 바이러스 백신 시장 : 투여 경로별
제19장 RSV 바이러스 백신 시장 : 대상 환자층별
제20장 RSV 바이러스 백신 시장 : 유통 채널별
제21장 RSV 바이러스 백신 시장 : 주요 지역별
제22장 시장 집중 분석 : 주요 참가 기업별
섹션 VII : 지리적 지역 시장 기회 분석
제23장 시장 기회 분석 : 북미
제24장 시장 기회 분석 : 유럽
섹션 VIII : 기타 독점적 인사이트
제25장 결론
제26장 주요 인사이트
섹션 IX : 부록
제27장 표 형식 데이터
제28장 기업 및 단체 일람
AJY 25.05.23RSV VACCINE MARKET
As per Roots Analysis, the global RSV vaccine market size is worth USD 1,149 million in the current year and is expected to be worth USD 731 million by 2040.
The opportunity for RSV vaccine market has been distributed across the following segments:
Type of Vaccine
- Subunit / Viral-like-Particle (VLP) Vaccines
- Live Attenuated Vaccines
- mRNA Vaccines
Route of Administration
- Intramuscular
- Other Routes
Target Patient Population
- Elderly Individuals
- Infants / Children
- Pregnant Individuals
Distribution Channel
- Pharmacies and Drug Stores
- Government / Institutional Suppliers
- Others
Key Geographical Regions
- North America
- Europe
RSV VACCINE MARKET: GROWTH AND TRENDS
Respiratory Syncytial Virus (RSV) is a common virus spreading lower respiratory tract infections in infants, older adults, and immunocompromised individuals. This virus is recognized as a highly contagious respiratory pathogen, which contributes to severe illness and mortality across the globe. According to the WHO, around 33 million new cases of RSV infections are emerging annually in children under the age of five across the globe. This can be attributed to the lack of effective treatment options for RSV infections.
Currently, several preventive measures, such as over-the-counter medications, nasal sprays, and humidifiers are being used for the treatment of RSV infections. However, they only provide symptomatic relief, making vaccines a promising alternative that offers safe, effective and targeted treatment to patients. These vaccines have the potential to minimize hospitalization rates, complications, and mortality rates related to RSV infections as well as other respiratory illnesses, such as bronchitis and pneumonia.

Driven by technological advancements, including the use of mRNA and nanoparticle technology for developing RSV vaccines, these vaccines are providing long lasting immunity and effective treatment against RSV infections. Moreover, owing to the increased research and development efforts and rising number of clinical trials for RSV treatment, the demand for RSV vaccines is anticipated to rise, positioning the market for healthy growth in the forthcoming years.
RSV VACCINE MARKET: KEY INSIGHTS
The report delves into the current state of the RSV vaccine market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Presently, more than 70 RSV vaccines are being developed using various technologies; notably, over 60% of these vaccines are under investigation in different clinical trial phases.
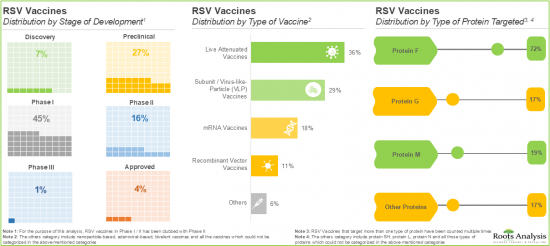
- The rising demand for RSV vaccines has led to a notable increase in the vaccine pipeline, with around 65 vaccines currently under preclinical and clinical stages of development.
- A sizeable increase in the number of registered clinical trials has been observed in recent years; over 85% of the trials evaluating RSV vaccines have been sponsored by industry players.
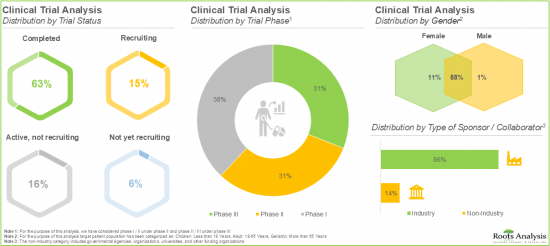
- Since 2020, more than 330 patents have been filed / granted in the RSV vaccines domain; further, the majority of the patents were filed in North America (>50%).
- The growing prevalence of respiratory syncytial virus infections, coupled with the increasing pace of technological advancements is driving the RSV vaccines market and positioning it for steady growth in the foreseeable future.
- The overall opportunity within the RSV vaccine market is anticipated to be well distributed across various key geographical regions, types of vaccines, routes of administration, target patient populations and distribution channels.
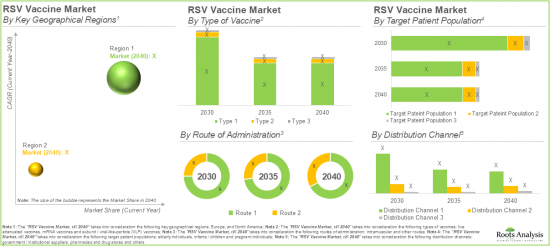
RSV VACCINE MARKET: KEY SEGMENTS
Subunit / Viral-Like-Particle (VLP) Vaccines are Likely to Dominate the RSV Vaccine Market During the Forecast Period
Based on the types of vaccines, the global RSV vaccine market is segmented into subunit / viral-like-particle (VLP) vaccines, live attenuated vaccines and mRNA vaccines. Currently, the majority share of the RSV vaccine market is captured by subunit / Viral-Like-Particle (VLP) vaccines, owing to the strong immunogenic response produced by these vaccines since they are morphologically similar to their parent virus.
Vaccines Administered through Intramuscular Route are Likely to Hold the Largest Share of the RSV Vaccine Market during the Forecast Period
Based on the route of administration, the global RSV vaccine market is distributed across intramuscular and other routes. Currently, the vaccines administered through the intramuscular route are likely to dominate the overall market. This can be attributed to their high bioavailability, which ensures efficient absorption, along with reduced side effects, making them a safer and more effective option for patients.
By Target Patient Population, RSV Vaccines Developed for Elderly Individuals are Likely to Dominate the Market during the Forecast Period
Based on the target patient population, the global RSV vaccine market is segmented into elderly individuals, infants / children and pregnant individuals. The current RSV vaccine market is likely to be dominated by elderly individuals' segment. This can be attributed to the fact that elderly individuals are highly susceptible to respiratory diseases, specifically during RSV season.
By Type of Material, Stainless Steel Segment is Likely to Dominate the Market During the Forecast Period
Based on the distribution channel, the global RSV vaccine market is segmented into pharmacies and drug stores, government / institutional suppliers and others. Notably, the pharmacies and drug stores segment are likely to dominate the RSV vaccine market during the forecast period. This can be attributed to their convenience and widespread accessibility, along with the rising authorization of pharmacists to administer vaccines which has contributed to increased vaccination rates.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America and Europe. In the current scenario, North America is likely to capture the largest market share which can be attributed to the growing incidences of respiratory infections, robust regulatory frameworks, and advanced healthcare infrastructure in this region.
Example Players in the RSV Vaccine Market
- AIM Vaccine
- Beijing Advaccine Biotechnology
- CureVac
- Daiichi Sankyo
- GlaxoSmithKline
- Icosavax (A company of AstraZeneca)
- Johnson & Johnson
- KM Biologics
- MedImmune (A part of AstraZeneca)
- Moderna
- Pfizer
- Sanofi
- SK Bioscience
RSV VACCINE MARKET: RESEARCH COVERAGE
The report on the RSV vaccine market features insights on various sections, including:
- Market Sizing and Opportunity Analysis: An in-depth analysis of the RSV vaccine market, focusing on key market segments, including [A] type of vaccine, [B] route of administration, [C] target patient population, [D] distribution channel, and [E] key geographical regions and [F] leading players.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
- RSV Vaccines Market Landscape: A comprehensive evaluation of various RSV vaccines, based on several relevant parameters, such as [A] stage of development, [B] route of administration, [C] type of vaccine, [D] type of protein targeted, [E] type of immunization, and [F] target patient population. This section also includes an evaluation of the companies engaged in developing RSV vaccines, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters.
- Product Competitiveness Analysis: An insightful competitiveness analysis of various RSV vaccines based on various relevant parameters, such as [A] developer strength, [B] product competitiveness, and [C] portfolio diversity.
- Company Profiles: Elaborate profiles of prominent RSV vaccine developers across various geographies, including North America, Europe, Asia-Pacific, providing details on [A] company overview, [B] financial information (if available), [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Clinical Trial Analysis: A detailed assessment of clinical trials that have been published for various types of RSV vaccines, based on various relevant parameters, such as [A] trial registration year, [B] trial status, [C] trial phase, [D] study design, [E] type of sponsor / collaborator, [F] gender, [G] target patient population, [H] leading players and [I] geography.
- Patent Analysis: An in-depth analysis of patents filed / granted till date in the RSV vaccine domain, based on various relevant parameters, such as [A] type of patent, [B] publication year, [C] patent application year, [D] CPC symbols, [E] patent jurisdiction, [F] type of applicant, [G] leading industry players, [H] leading industry players, [I] patent benchmarking, [J] patent age, and [K] patent valuation analysis.
- FDA Approval Strategies: A detailed overview of various competitive strategies that can be incorporated by the RSV vaccine developers in order to expedite the FDA approval process of their proprietary vaccines. The section also provides information on several stakeholders undertaking various initiatives, including awards / grants, partnerships, and expanding intellectual properties.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
- What is the patent filing activity trend in the market?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
SECTION I: REPORT OVERVIEW
1. BACKGROUND
- 1.1. Context
- 1.2. Project Objectives
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
- 2.5. Robust Quality Control
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Forecast Methodology
- 3.2.1. Top-down Approach
- 3.2.2. Bottom-up Approach
- 3.2.3. Hybrid Approach
- 3.3. Market Assessment Framework
- 3.3.1. Total Addressable Market (TAM)
- 3.3.2. Serviceable Addressable Market (SAM)
- 3.3.3. Serviceable Obtainable Market (SOM)
- 3.3.4. Currently Acquired Market (CAM)
- 3.4. Forecasting Tools and Techniques
- 3.4.1. Qualitative Forecasting
- 3.4.2. Correlation
- 3.4.3. Regression
- 3.4.4. Extrapolation
- 3.4.5. Convergence
- 3.4.6. Sensitivity Analysis
- 3.4.7. Scenario Planning
- 3.4.8. Data Visualization
- 3.4.9. Time Series Analysis
- 3.4.10. Forecast Error Analysis
- 3.5. Key Considerations
- 3.5.1. Demographics
- 3.5.2. Government Regulations
- 3.5.3. Reimbursement Scenarios
- 3.5.4. Market Access
- 3.5.5. Supply Chain
- 3.5.6. Industry Consolidation
- 3.5.7. Pandemic / Unforeseen Disruptions Impact
- 3.6. Limitations
4. MACRO-ECONOMIC INDICATORS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Forecasted Estimates
- 4.2.2. Currency Coverage
- 4.2.2.1. Major Currencies Affecting the Market
- 4.2.2.2. Factors Affecting Currency Fluctuations in the Industry
- 4.2.2.3. Impact of Currency Fluctuations on the Industry
- 4.2.3. Foreign Currency Exchange Rate
- 4.2.3.1. Impact of Foreign Exchange Rate Volatility on the Market
- 4.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 4.2.4. Recession
- 4.2.4.1. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.4.2. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.6. Interest Rates
- 4.2.6.1. Interest Rates and Their Impact on the Market
- 4.2.6.2. Strategies for Managing Interest Rate Risk
- 4.2.7. Commodity Flow Analysis
- 4.2.7.1. Type of Commodity
- 4.2.7.2. Origins and Destinations
- 4.2.7.3. Values and Weights
- 4.2.7.4. Modes of Transportation
- 4.2.8. Global Trade Dynamics
- 4.2.8.1. Import Scenario
- 4.2.8.2. Export Scenario
- 4.2.8.3. Trade Policies
- 4.2.8.4. Strategies for Mitigating the Risks Associated with Trade Barriers
- 4.2.8.5. Impact of Trade Barriers on the Market
- 4.2.9. War Impact Analysis
- 4.2.9.1. Russian-Ukraine War
- 4.2.9.2. Israel-Hamas War
- 4.2.10. COVID Impact / Related Factors
- 4.2.10.1. Global Economic Impact
- 4.2.10.2. Industry-specific Impact
- 4.2.10.3. Government Response and Stimulus Measures
- 4.2.10.4. Future Outlook and Adaptation Strategies
- 4.2.11. Other Indicators
- 4.2.11.1. Fiscal Policy
- 4.2.11.2. Consumer Spending
- 4.2.11.3. Gross Domestic Product
- 4.2.11.4. Employment
- 4.2.11.5. Taxes
- 4.2.11.6. Stock Market Performance
- 4.2.11.7. Cross Border Dynamics
- 4.2.1. Time Period
- 4.3. Conclusion
SECTION II: QUALITATIVE INSIGHTS
5. EXECUTIVE SUMMARY
6. INTRODUCTION
- 6.1. Introduction to RSV Vaccines
- 6.2. Structure and Mechanism of RSV
- 6.3. Key Historical Events Related to RSV Vaccines
- 6.4 Types of RSV Vaccines
- 6.4.1. Nucleic Acid Based Vaccines
- 6.4.2. Protein Based Vaccines
- 6.4.3. Recombinant Vector Based Vaccines
- 6.4.4. Live Attenuated Vaccines
- 6.5. Benefits of RSV Vaccination
- 6.6. Challenges Associated with Development of RSV Vaccines
- 6.7. Future Perspectives
SECTION III: MARKET OVERVIEW
7. MARKET LANDSCAPE
- 7.1. RSV Vaccines: Overall Market Landscape
- 7.1.1. Analysis by Stage of Development
- 7.1.2. Analysis by Route of Administration
- 7.1.3. Analysis by Type of Vaccine
- 7.1.4. Analysis by Type of Protein Targeted
- 7.1.5. Analysis by Type of Immunization
- 7.1.6. Analysis by Target Patient Population
- 7.1.7. Analysis by Year of Establishment
- 7.1.8. Analysis by Company Size
- 7.1.9. Analysis by Location of Headquarters
8. PRODUCT COMPETITIVENESS ANALYSIS
- 8.1. Assumptions and Key Parameters
- 8.2. Methodology
- 8.3. RSV Vaccines: Product Competitiveness Analysis
- 8.3.1. RSV Vaccines Developed by Players Based in North America
- 8.3.2. RSV Vaccines Developed by Players Based in Europe
- 8.3.3. RSV Vaccines Developed by Players Based in Asia-Pacific and Rest of the World
SECTION IV: COMPANY PROFILES
9. COMPANY PROFILES: RSV VACCINE DEVELOPERS IN NORTH AMERICA
- 9.1. Icosavax (A Company of AstraZeneca)
- 9.1.1. Company Details
- 9.1.2. Product Portfolio
- 9.1.3. Recent Developments and Future Outlook
- 9.2. Moderna
- 9.2.1. Company Details
- 9.2.2. Product Portfolio
- 9.2.3. Financial Details, FY 2021 onwards (USD Million)
- 9.2.4. Recent Developments and Future Outlook
- 9.3. Pfizer
- 9.3.1. Company Details
- 9.3.2. Product Portfolio
- 9.3.3. Financial Details, FY 2021 onwards (USD Million)
- 9.3.4. Recent Developments and Future Outlook
10. COMPANY PROFILES: RSV VACCINE DEVELOPERS IN EUROPE
- 10.1. GlaxoSmithKline
- 10.1.1. Company Details
- 10.1.2. Product Portfolio
- 10.1.3. Financial Details, FY 2021 onwards (EUR Million)
- 10.1.4. Recent Developments and Future Outlook
- 10.2. Sanofi
- 10.2.1. Company Details
- 10.2.2. Product Portfolio
- 10.2.3. Financial Details, FY 2021 onwards (EUR Million)
- 10.2.4. Recent Developments and Future Outlook
11. COMPANY PROFILES: RSV VACCINE DEVELOPERS IN ASIA-PACIFIC
- 11.1. Beijing Advaccine Biotechnology
- 11.1.1. Company Details
- 11.1.2. Product Portfolio
SECTION V: MARKET TRENDS
12. CLINICAL TRIALS ANALYSIS
- 12.1. Scope and Methodology
- 12.2. RSV Vaccines: Clinical Trial Analysis
- 12.2.1. Analysis by Trial Registration Year, Since 2015
- 12.2.2. Analysis by Trial Status
- 12.2.3. Analysis of Enrolled Patient Population by Trial Registration Year, Since 2015
- 12.2.4. Analysis by Trial Registration Year and Trial Status, Since 2015
- 12.2.5. Analysis by Trial Phase
- 12.2.6. Analysis of Enrolled Patient Population by Trial Phase
- 12.2.7. Analysis by Study Design
- 12.2.8. Analysis by Type of Sponsor / Collaborator
- 12.2.9. Analysis by Gender
- 12.2.10. Analysis by Target Patient Population
- 12.2.11. Leading Players: Analysis by Number of Registered Trials
- 12.2.12. Analysis by Geography
- 12.2.13. Analysis by Trial Status and Geography
- 12.2.14. Analysis of Enrolled Patient Population by Trial Status and Geography
13. PATENT ANALYSIS
- 13.1. Scope and Methodology
- 13.2. RSV Vaccines: Patent Analysis
- 13.2.1. Analysis by Patent Publication Year, Since 2020
- 13.2.2. Analysis by Type of Patent
- 13.2.3. Analysis by Type of Patent and Publication Year, Since 2020
- 13.2.4. Analysis by Patent Application Year, Since 2008
- 13.2.5. Analysis by Patent Age
- 13.2.6. Analysis by Type of Applicant
- 13.2.7. Analysis by Patent Jurisdiction
- 13.2.8. Analysis by CPC Symbols
- 13.2.9. Leading Industry Players: Analysis by Number of Patents
- 13.2.10. Leading Non-Industry Players: Analysis by Number of Patents
- 13.2.11. Leading Inventors: Analysis by Number of Patents
- 13.3. Patent Benchmarking Analysis
- 13.3.1. Analysis of Patent Characteristics (CPC Codes) by Leading Industry Players
- 13.3.2. Analysis of Leading Industry Players by Patent Characteristics (CPC Codes)
- 13.4. Patent Valuation
- 13.5. Leading Patents by Number of Citations
14. FDA APPROVAL STRATEGIES
- 14.1. Chapter Overview
- 14.2. Methodology
- 14.3. Key Parameters
- 14.4. General Reasons for Failure of Trials Focused on RSV vaccines
- 14.5. Benchmarking Analysis: Distribution of Key Strategies by Vaccines
SECTION VI: MARKET OPPORTUNITY ANALYSIS
15. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 15.1. Market Drivers
- 15.2. Market Restraints
- 15.3. Market Opportunities
- 15.4. Market Challenges
16. GLOBAL RSV VACCINE MARKET
- 16.1. Key Assumptions and Methodology
- 16.2. Global RSV Vaccine Market, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- 16.2.1. Multivariate Scenario Analysis
- 16.2.1.1. Conservative Scenario
- 16.2.1.2. Optimistic Scenario
- 16.2.1. Multivariate Scenario Analysis
- 16.3. Key Market Segmentations
17. RSV VACCINE MARKET, BY TYPE OF VACCINE
- 17.1. Key Assumptions and Methodology
- 17.2. RSV Vaccine Market: Distribution by Type of Vaccine
- 17.2.1. RSV Vaccine Market for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 17.2.2. RSV Vaccine Market for Live Attenuated Vaccines, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 17.2.3. RSV Vaccine Market for mRNA Vaccines, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 17.3. Data Triangulation and Validation
18. RSV VACCINE MARKET, BY ROUTE OF ADMINISTRATION
- 18.1. Key Assumptions and Methodology
- 18.2. RSV Vaccine Market: Distribution by Route of Administration
- 18.2.1. RSV Vaccine Market for Intramuscular, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 18.2.2. RSV Vaccine Market for Other Routes, Historical Trends (Since2023) and Forecasted Estimates Till 2040) (USD Million)
- 18.3. Data Triangulation and Validation
19. RSV VACCINE MARKET, BY TARGET PATIENT POPULATION
- 19.1. Key Assumptions and Methodology
- 19.2. RSV Vaccine Market: Distribution by Target Patient Population
- 19.2.1. RSV Vaccine Market for Elderly Individuals, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 19.2.2. RSV Vaccine Market for Infants / Children, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 19.2.3. RSV Vaccine Market for Pregnant Individuals, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 19.3. Data Triangulation and Validation
20. RSV VACCINE MARKET, BY DISTRIBUTION CHANNEL
- 20.1. Key Assumptions and Methodology
- 20.2. RSV Vaccine Market: Distribution by Distribution Channel
- 20.2.1. RSV Vaccine Market for Pharmacies and Drug Stores, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 20.2.2. RSV Vaccine Market for Government / Institutional Suppliers, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 20.2.3. RSV Vaccine Market for Others, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 20.3. Data Triangulation and Validation
21. RSV VACCINE MARKET, BY KEY GEOGRAPHICAL REGIONS
- 21.1. Key Assumptions and Methodology
- 21.2. RSV Vaccine Market: Distribution by Key Geographical Regions
- 21.2.1. RSV Vaccine Market in North America, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 21.2.2. RSV Vaccine Market in Europe, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 21.3. Market Movement Analysis
- 21.4. Penetration-Growth (P-G) Matrix
- 21.5. Data Triangulation and Validation
22. MARKET CONCENTRATION ANALYSIS: DISTRIBUTION BY LEADING PLAYERS
- 22.1. Key Assumptions and Methodology
- 22.2. RSV Vaccine Market: Leading RSV Vaccines Developers
- 22.3. Data Triangulation and Validation
SECTION VII: MARKET OPPORTUNITY ANALYSIS WITHIN GEOGRAPHICAL REGIONS**
23. MARKET OPPORTUNITY ANALYSIS: NORTH AMERICA
- 23.1. RSV Vaccine Market in North America: Distribution by Type of Vaccine
- 23.1.1. RSV Vaccine Market in North America for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.1.2. RSV Vaccine Market in North America for Live Attenuated Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.1.3. RSV Vaccine Market in North America for mRNA Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.2. RSV Vaccine Market in North America: Distribution by Route of Administration
- 23.2.1. RSV Vaccine Market in North America for Intramuscular, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.2.2. RSV Vaccine Market in North America for Other Routes, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.3. RSV Vaccine Market in North America: Distribution by Target Patient Population
- 23.3.1. RSV Vaccine Market in North America for Elderly Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.3.2. RSV Vaccine Market in North America for Infants / Children, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.3.3. RSV Vaccine Market in North America for Pregnant Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.4. RSV Vaccine Market in North America: Distribution by Distribution Channel
- 23.4.1. RSV Vaccine Market in North America for Pharmacies and Drug Stores, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.4.2. RSV Vaccine Market in North America for Government / Institutional Suppliers, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.4.3. RSV Vaccine Market in North America for Others, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
24. MARKET OPPORTUNITY ANALYSIS: EUROPE
- 24.1. RSV Vaccine Market in Europe: Distribution by Type of Vaccine
- 24.1.1. RSV Vaccine Market in Europe for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.1.2. RSV Vaccine Market in Europe for Live Attenuated Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.1.3. RSV Vaccine Market in Europe for mRNA Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.2. RSV Vaccine Market in Europe: Distribution by Route of Administration
- 24.2.1. RSV Vaccine Market in Europe for Intramuscular, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.2.2. RSV Vaccine Market in Europe for Other Routes, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.3. RSV Vaccine Market in Europe: Distribution by Target Patient Population
- 24.3.1. RSV Vaccine Market in Europe for Elderly Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.3.2. RSV Vaccine Market in Europe for Infants / Children, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.3.3. RSV Vaccine Market in Europe for Pregnant Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.4. RSV Vaccine Market in Europe: Distribution by Distribution Channel
- 24.4.1. RSV Vaccine Market in Europe for Pharmacies and Drug Stores, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.4.2. RSV Vaccine Market in Europe for Government / Institutional Suppliers, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.4.3. RSV Vaccine Market in Europe for Others, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- *Detailed information on Section VII is available in the Excel Data Packs shared along with the report**



















