
|
시장보고서
상품코드
1737047
동종 세포 치료 시장 : 세포 요법 유형별, 세포 유래별, 표적 적응증별, 치료 영역별, 주요 지역별Allogeneic Cell Therapy Market by Type of Cell Therapy, Source of Cell, Target Indication, Therapeutic Area, and Key Geographical Regions |
||||||
세계 동종세포 치료 시장 규모는 2035년까지 예측 기간 동안 5.9%의 연평균 복합 성장률(CAGR)로, 9억 8,000만 달러에서 2035년까지 27억 4,000만 달러로 성장할 것으로 예측됩니다.
시장 세분화는 시장 규모와 기회 분석을 다음 매개 변수로 구분합니다.
세포 치료 유형별
- 줄기세포
- 바이러스 특이적 T 세포
- 제어성 T 세포
세포 유래별
- 골수/매트릭스
- 지방조직
- 말초혈
- 제대
- 태반
- 기타
표적 적응증별
- 감염증
- 만성 심부전
- 크론병
- 허혈성 뇌졸중
- 혈액암
- 뼈 질환
- 중증 허혈지
- 이식편 대숙주병
- 두첸느형 근이영양증
- 표피 수포증
- 당뇨병성 발궤양
- 기타
치료 영역별
- 이식 후 감염증
- 심혈관 질환
- 자가면역/염증성 질환
- 뇌혈관 장애
- 종양학적 질환
- 근골격계 장애
- 신경질환
- 유전성 질환
- 기타
지역별
- 북미
- 유럽
- 아시아태평양, 기타
동종 세포 치료 시장 : 성장과 동향
세포치료란 손상된 조직을 보충하거나 직접적인 상호작용이나 인자의 발현을 통해 세포의 기능을 조절하기 위해 환자의 체내에 인공세포를 투여하는 것을 말합니다.
동종 세포 치료은 종종 "기성품"세포 요법이라고 불리며 환자 자신이 아닌 기증자의 세포를 사용하는 재생 의학의 한 형태입니다. 환자 자신의 세포를 이용한 자가세포요법과는 달리, 동종세포요법에는 몇 가지 명확한 이점이 있습니다. Alliance for Regenerative Medicine에 따르면 동종세포를 이용한 암치료의 임상시험수는 지난 5년간 30% 증가했습니다. 또한 이들 세포는 치료특성을 높이기 위해 유전자 변형이 가능하고 질병세포를 표적으로 파괴하거나 조직의 수복을 촉진하는 효과가 더 높아지는 것도 특필할 만합니다.
동종 세포 치료 시장 : 주요 인사이트력
이 보고서는 동종 세포 치료 시장의 현재 상태를 파악하고 이 산업의 잠재적 성장 기회를 보여줍니다.
- 480개 이상의 동종세포요법이 190개 이상의 기업별 승인 또는 조사 중이며, 시장정세에는 기존기업과 신규 참가기업 모두 존재합니다.
- 임상단계에 있는 동종세포요법의 대부분은 제II상시험에서 평가되고 있습니다.
- 현재, 365건 이상의 임상시험(등록 환자수 18,000명 이상)이 진행중이며, 다양한 지역에서 동종 세포 치료이 검토되고 있습니다.
- 이 분야에 대한 이해관계자의 관심이 높아지고 있는 것은 동종세포치료개발자가 국제적 및 국내적인 다양한 파트너와 폭넓은 파트너십을 맺고 있는 것에도 반영되고 있습니다.
- 이 분야의 기회를 깨달은 여러 투자자는 지난 5년간 약 88억 달러(120개의 자금조달 사례에 걸쳐)를 투자하고 있으며, 그 최고액은 벤처캐피탈 라운드를 통해 투자된 것입니다.
- 학술/의료/상업 단체에 소속된 다양한 KOL이 다양한 질병 적응에 있어서의 동종 세포 치료의 효율을 평가했습니다.
- 만성질환의 이환율 상승과 신규 동종세포요법에 대한 수요 증가에 견인되어, 이 시장은 2035년까지 연평균 복합 성장률(CAGR)은 5.9%를 나타낼 것으로 예측됩니다.
- 동종 세포 치료의 미래 기회는 다양한 유형의 적응증, 치료 영역, 주요 지리적 지역에 분산되어있을 것으로 예측됩니다.
동종 세포 치료 시장 : 주요 부문
세포요법 유형별로 동종세포요법 세계 시장은 줄기세포요법, 바이러스 특이적 T세포요법, 제어성 T세포요법으로 구분됩니다.
세포 유래별로 시장은 골수/매트릭스, 지방조직, 말초혈, 제대, 태반 등으로 구분됩니다. 종세포 치료 시장에서 가장 높은 비율을 차지합니다. 지방 조직 유래 치료 부문의 동종 세포 치료 세계 시장은 상대적으로 높은 CAGR로 성장할 가능성이 높습니다.
표적 적응증별로, 시장은 감염증, 만성 심부전, 크론병, 허혈성 뇌졸중, 혈액암, 골질환, 중증 허혈지, 이식편 대숙주병, 두첸느형 근이영양증, 표피수포증, 당뇨병성 발궤양 등 구분됩니다. 현재, 감염증 분야가 세계의 동종 세포 치료 시장의 대부분을 차지하고 있습니다.
치료 영역별로 보면, 동종세포 치료 세계 시장은 이식 후 감염증, 심혈관 장애, 자가면역/염증성 질환, 뇌혈관 장애, 종양성 질환, 근골격계 질환, 신경 질환, 유전성 질환, 등에 분포하고 있습니다. 재이식 후 감염증이나 심혈관질환에 대한 동종세포요법이 시장 전체를 지배하고 있습니다.
주요 지역별로 볼 때 시장은 북미, 유럽, 아시아태평양 및 기타 아시아태평양으로 구분됩니다. 현재 북미가 가장 큰 시장 점유율을 차지하고 있습니다.
동종세포요법시장 진입기업 예
- Artiva Biotherapeutics
- Allogene Therapeutics
- Atara Biotherapeutics
- Cellenkos
- Cell2Cure
- Celularity
- Cellular Biomedicine Group
- CHABiotech
- CRISPR Therapeutics
- Fate Therapeutics
- Fundamenta Therapeutics
- GC Cell
- Hope Biosciences
- Immunity Bio
- Mesoblast
- Nanjing Bioheng Biotech
- Orca Bio
- Pluristem Therapeutics
- Poseida Therapeutics
- Stemedica Cell Technologies
본 보고서에서는 세계의 동종세포요법 시장에 대해 조사했으며,, 시장 개요와 함께 세포치료 유형별, 세포유래별, 표적적응증별, 치료영역별, 주요지별 동향, 시장 진출기업 프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제1장 동종 세포 치료 시장 개요
제2장 주요 요약
제3장 소개
- 동종세포요법의 개요
- 동종 세포 치료의 제조 공정
- 동종세포요법의 장점
- 동종 세포 치료에 따른 과제
- 동종세포치료산업의 최근 동향
- 장래의 전망
제4장 시장 상황
- 동종 세포 치료 : 시장 상황
- 개발 단계별 분석
- 투여 경로별 분석
- 세포치료유형별 분석
- 세포원별 분석
- 투여 빈도별 분석
- 치료법별 분석
- 대상 환자 부문별 분석
- 대상 적응증별 분석
- 치료 영역별 분석
- 동종 세포 치료 : 개발 상황
- 설립년별 분석
- 기업 규모별 분석
- 본사 소재지별 분석
- 가장 활발한 진출기업 : 치료 수별 분석
제5장 파트너십 및 협업
- 장의 개요
- 파트너십 모델
- 동종세포요법: 파트너십 및 협업
제6장 자금 조달과 투자
- 장의 개요
- 자금 조달의 유형
- 동종 세포 치료 : 자금 조달 및 투자
제7장 임상시험의 분석
- 분석 조사 방법과 주요 파라미터
- 동종세포요법: 임상시험 분석
제8장 KOL(Key Opinion Leader)
- 조사 방법과 주요 파라미터
- 동종세포요법: 키오피니언 리더(KOL)
제9장 시장 예측과 기회 분석
- 장의 개요
- 주요 전제와 조사 방법
- 세계의 동종 세포 치료 시장(2035년까지)
- 동종세포 치료 시장 : 제품별 매출 예측(2035년까지)
- 레바스콜/MPC-150-IM/렉세메스트로셀-L
- 알로피셀/달바도스트로셀/Cx601
- 멀티스템(R)(어서시스)
- 탭 셀(R)/탭 셀 셀/ATA129
- MDR-101
- PLX 패드
- OMISIRGE/오미두비셀-onlv
- 오르카 T
- 리온실
- 스템 퓨셀(R)
- 빌라림 M/ALVR105/포솔레우셀
- 트리니티 에볼루션(R)
- CAP-1002
- 오스테오셀 플러스
- MPC-06-ID/렉세메스트로셀-L
- AB-205/E-CEL 세포
- 알로-APZ2-OTS
- 트리니티 엘리트
- CYP-004
- 사이토빌 CMV T 세포
- 까르띠스템(R)
- 그래픽(R)
- 엘릭스 사이트
- 템셀(R)HS
- 알로 ASC-DFU
제10장 주요 인사이트
제11장 부록 I: 표 형식 데이터
제12장 부록 I1: 기업 및 조직 목록
SHW 25.06.09ALLOGENEIC CELL THERAPY MARKET: OVERVIEW
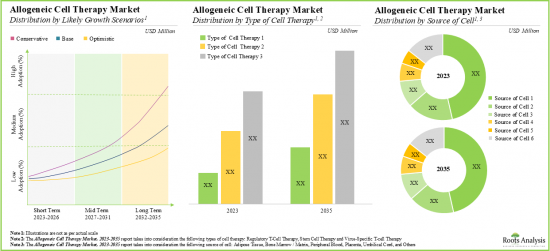
As per Roots Analysis, the global allogenic cell therapy market is estimated to grow from USD 0.98 billion in the current year to USD 2.74 billion by 2035, at a CAGR of 5.9% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Cell Therapy
- Stem Cell
- Virus-Specific T-Cell
- Regulatory T-Cell
Source of Cell
- Bone Marrow / Matrix
- Adipose Tissue
- Peripheral Blood
- Umbilical Cord
- Placenta
- Others
Target Indication
- Infectious Diseases
- Chronic Heart Failure
- Crohn's Disease
- Ischemic Stroke
- Hematological Cancer
- Bone Diseases
- Critical Limb Ischemia
- Graft versus Host Disease
- Duchenne Muscular Dystrophy
- Epidermolysis Bullosa
- Diabetic Foot Ulcer
- Others
Therapeutic Area
- Post-Transplant Infections
- Cardiovascular Disorders
- Autoimmune / Inflammatory Disorders
- Cerebrovascular Disorders
- Oncological Disorders
- Musculoskeletal Disorders
- Neurological Disorders
- Genetic Disorders
- Others
Key Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
ALLOGENEIC CELL THERAPY MARKET: GROWTH AND TRENDS
Cell therapy refers to the administration of engineered cells into a patient's body in order to replace the damaged tissues or to modulate the cell functioning through direct interaction or expression of factors. Depending on its source, cell therapies can either be autologous (derived from the patient's own body) or allogeneic (derived from a healthy donor).
Allogenic cell therapy, often referred to as "off-the-shelf" cell therapy, is a form of regenerative medicine that involves the use of cells from a donor, rather than the patient themselves. These cells can be derived from various sources, such as bone marrow, umbilical cord blood, or induced pluripotent stem cells (iPSCs). Unlike autologous cell therapy, where a patient's own cells are used, allogenic cell therapy provides several distinct advantages. One of the key applications of allogeneic cell therapy is in the treatment of various diseases, such as cancer, autoimmune disorders, and degenerative conditions. In fact, according to the Alliance for Regenerative Medicine, the number of clinical trials being conducted for allogeneic cell-based cancer therapies increased by 30% over the past five years. Further, it is worth mentioning that these cells can be genetically modified to enhance their therapeutic properties, making them more effective at targeting and destroying disease cells or promoting tissue repair.
ALLOGENEIC CELL THERAPY MARKET: KEY INSIGHTS
The report delves into the current state of the allogeneic cell therapy market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Over 480 allogeneic cell therapies are either approved or being investigated in research studies by more than 190 players; the market landscape features the presence of both established players and new entrants.
- The majority of the clinical-stage allogeneic cell therapies are being evaluated in phase II trials; these therapies are being derived from different sources of cells and target multiple disease indications.

- More than 365 clinical trials (with over 18,000 enrolled patients) are currently underway to investigate allogeneic cell therapies across different geographies.
- The rising interest of stakeholders in this domain is reflected in the wide array of partnerships established by allogeneic cell therapy developers with various international and indigenous partners.
- Having realized the opportunity in this segment, several investors have invested around USD 8.8 billion (across 120 funding instances), in the past five years; the maximum amount was invested through venture capital rounds.
- Various KOLs affiliated to academic / medical / commercial organizations are evaluating the efficiency of allogeneic cell therapies in different disease indications.
- Driven by the rising incidence of chronic diseases and growing demand for novel allogeneic cell therapies, this market is anticipated to grow at a CAGR of 5.9%, till 2035.

- The projected future opportunity for allogeneic cell therapies is likely to be well distributed across different types of target indications, therapeutic areas and key geographical regions.
ALLOGENEIC CELL THERAPY MARKET: KEY SEGMENTS
Regulatory T-Cell Therapies is the Fastest Growing Segment of the Allogeneic Cell Therapy Market
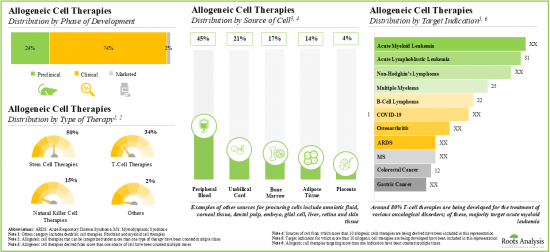
Based on the type of cell therapy, the global market for allogeneic cell therapy is segmented into stem cell, virus-specific T cell and regulatory T-cell therapies. Currently, the majority share of the allogeneic cell therapy market is captured by stem cell therapies. It is worth mentioning that the regulatory T-cell therapies segment is likely to grow at a higher CAGR (~ 23%) in the coming years.
By Source of Cell, Bone Marrow / Matrix Segment Dominates the Global Allogeneic Cell Therapy Market
Based on the source of cells, the market is segmented into bone marrow / matrix, adipose tissue, peripheral blood, umbilical cord, placenta and others. Currently, the market is dominated by bone marrow / matrix-derived allogenic cell therapies, capturing the highest proportion of the allogeneic cell therapy market. It is worth highlighting that the global allogeneic cell therapy market for the adipose tissue-derived therapies segment is likely to grow at a relatively higher CAGR.
Infectious Diseases Segment is Likely to Hold the Largest Share of the Allogeneic Cell Therapy Market During the Forecast Period
Based on the target indication, the market is segmented into infectious diseases, chronic heart failure, Crohn's disease, ischemic stroke, hematological cancer, bone disease, critical limb ischemia, graft versus host disease, Duchenne muscular dystrophy, epidermolysis bullosa, diabetic foot ulcer and others. At present, the infectious diseases segment holds the majority share of the global allogeneic cell therapy market. Notably, epidermolysis bullosa target indication segment is likely to grow at a relatively higher CAGR.
By Therapeutic Area, Allogeneic Cell Therapies for Post-Transplant Infections are Likely to Dominate the Market During the Forecast Period
Based on the therapeutic area, the global market for allogeneic cell therapy is distributed across post-transplant infections, cardiovascular disorders, autoimmune / inflammatory disorders, cerebrovascular disorders, oncological disorders, musculoskeletal disorders, neurological disorders, genetic disorders and others. Currently, allogeneic cell therapies for post-transplant infections and cardiovascular disorders dominate the overall market. However, the market for cell therapies used in autoimmune / inflammatory disorders is likely to grow at a relatively higher CAGR.
North America Accounts for the Largest Share of the Market
Based on the key geographical regions, the market is segmented into North America, Europe, and Asia-Pacific and Rest of the World. In the current scenario, North America is likely to capture the largest market share.
Example Players in the Allogeneic Cell Therapy Market
- Artiva Biotherapeutics
- Allogene Therapeutics
- Atara Biotherapeutics
- Cellenkos
- Cell2Cure
- Celularity
- Cellular Biomedicine Group
- CHABiotech
- CRISPR Therapeutics
- Fate Therapeutics
- Fundamenta Therapeutics
- GC Cell
- Hope Biosciences
- Immunity Bio
- Mesoblast
- Nanjing Bioheng Biotech
- Orca Bio
- Pluristem Therapeutics
- Poseida Therapeutics
- Stemedica Cell Technologies
ALLOGENEIC CELL THERAPY MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global allogeneic cell therapy market, focusing on key market segments, including [A] type of cell therapy, [B] source of cell, [C] target indication, [D] therapeutic area and [E] key geographical regions.
- Allogeneic Cell Therapies Market Landscape: A comprehensive evaluation of allogeneic cell therapies, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] phase of development, [E] route of administration, [F] type of cell therapy, [G] source of cell, [H] dosing frequency, [I] type of therapy, [J] target patient segment, [K] target indication and [L] therapeutic area.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in allogeneic cell therapy domain, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of cell therapy, [D] therapeutic area, [E] type of partner, [F] most active players and [G] geographical distribution of partnership activity.
- Funding and Investment Analysis: An in-depth analysis of the fundings raised by companies engaged in this domain, based on relevant parameters, such as [A] year of investment, [B] amount invested, [C] type of funding, [D] type of investor, [E] type of therapy, [F] geographical distribution and [G] most active players.
- Clinical Trial Analysis: An insightful analysis of clinical trials related to allogeneic cell therapies, based on several parameters, such as [A] trial registration year, [B] trial status, [C] trial phase, [D] study design, [E] type of sponsor, [F] geography and [G] most active industry and non-industry players.
- Key Opinion Leaders (KOLs]: An in-depth analysis that emphasizes the key opinion leaders investigating clinical trials related to allogeneic cell therapies, considering various parameters, such as [A] type of KOL, [B] qualification, [C] type of organization, [D] affiliated organization, [E] geographical location of KOLs and [F] target disease indication.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
1.1. Allogeneic Cell Therapy Market Overview
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Research Methodology
- 1.5. Frequently Asked Questions
- 1.6. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Overview of Allogeneic Cell Therapy
- 3.2. Manufacturing Process of Allogeneic Cell Therapy
- 3.3. Advantages Offered by Allogeneic Cell Therapy
- 3.4. Challenges Associated with Allogeneic Cell Therapy
- 3.5. Recent Developments in Allogeneic Cell Therapy Industry
- 3.6. Future Perspectives
4. MARKET LANDSCAPE
- 4.1. Allogeneic Cell Therapies: Market Landscape
- 4.1.1. Analysis by Phase of Development
- 4.1.2. Analysis by Route of Administration
- 4.1.3. Analysis by Type of Cell Therapy
- 4.1.4. Analysis by Source of Cell
- 4.1.5. Analysis by Dosing Frequency
- 4.1.6. Analysis by Type of Therapy
- 4.1.7. Analysis by Target Patient Segment
- 4.1.8. Analysis by Target Indication
- 4.1.9. Analysis by Therapeutic Area
- 4.2. Allogeneic Cell Therapies: Developer Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Most Active Players: Analysis by Number of Therapies
5. PARTNERSHIPS AND COLLABORATIONS
- 5.1. Chapter Overview
- 5.2. Partnership Models
- 5.3. Allogeneic Cell Therapies: Partnerships and Collaborations
- 5.3.1. Analysis by Year of Partnership
- 5.3.2. Analysis by Type of Partnership
- 5.3.3. Analysis by Year and Type of Partnership
- 5.3.4. Analysis by Type of Cell Therapy
- 5.3.5. Analysis by Therapeutic Area
- 5.3.6. Analysis by Type of Partner
- 5.3.7. Most Active Players: Analysis by Number of Partnerships
- 5.3.8. Analysis by Geography
- 5.3.8.1. Intercontinental and Intracontinental Deals
- 5.3.8.2. Local and International Deals
6. FUNDING AND INVESTMENTS
- 6.1. Chapter Overview
- 6.2. Types of Funding
- 6.3. Allogeneic Cell Therapies: Funding and Investments
- 6.3.1. Analysis by Year of Investment
- 6.3.2. Analysis by Amount Invested
- 6.3.3. Analysis by Type of Funding
- 6.3.4. Analysis of Amount Invested by Type of Funding
- 6.3.5. Analysis of Amount Invested by Year and Type of Funding
- 6.3.6. Analysis by Type of Investor
- 6.3.7. Analysis by Type of Therapy
- 6.3.8. Analysis by Geography
- 6.3.9. Leading Investors: Analysis by Number of Instances
- 6.3.10. Most Active Players: Analysis by Number of Instances
- 6.3.11. Most Active Players: Analysis by Amount Invested
7. CLINICAL TRIAL ANALYSIS
- 7.1. Analysis Methodology and Key Parameters
- 7.2. Allogeneic Cell Therapies: Clinical Trial Analysis
- 7.2.1. Analysis by Trial Registration Year
- 7.2.2. Analysis by Trial Status
- 7.2.3. Analysis by Trial Registration Year and Trial Status
- 7.2.4. Analysis by Trial Registration Year and Patients Enrolled
- 7.2.5. Analysis by Trial Status and Patients Enrolled
- 7.2.6. Analysis by Trial Phase
- 7.2.7. Analysis by Study Design
- 7.2.8. Analysis by Trial Status, Trial Phase and Geography
- 7.2.9. Analysis by Type of Sponsor
- 7.2.10. Most Active Industry Players: Analysis by Number of Trials
- 7.2.11. Most Active Non-Industry Players: Analysis by Number of Trials
- 7.2.12. Analysis by Geography
8. KEY OPINION LEADERS
- 8.1. Methodology and Key Parameters
- 8.2. Allogeneic Cell Therapies: Key Opinion Leaders (KOLs)
- 8.2.1. Analysis by Type of KOL
- 8.2.2. Analysis by Qualification
- 8.2.3. Analysis by Type of Organization
- 8.2.4. Analysis by Affiliated Organization
- 8.2.5. Analysis by Target Disease Indication
- 8.2.6. Analysis by Geographical Location of KOLs
- 8.2.7. Most Prominent KOLs: Peer Group 1 (Principal Investigators)
- 8.2.8. Most Prominent KOLs: Peer Group 2 (Study Directors)
- 8.2.9. Most Prominent KOLs: Peer Group 3 (Study Chair)
- 8.2.10. Most Prominent KOLs: Analysis by RA Score
9. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 9.1. Chapter Overview
- 9.2. Key Assumptions and Methodology
- 9.3. Global Allogeneic Cell Therapy Market, Till 2035
- 9.3.1. Allogeneic Cell Therapy Market: Distribution by Type of Cell Therapy, Till 2035
- 9.3.1.1. Allogeneic Cell Therapy Market for Stem Cell Therapies, Till 2035
- 9.3.1.2. Allogeneic Cell Therapy Market for Virus-specific T-cell Therapies, Till 2035
- 9.3.1.3. Allogeneic Cell Therapy Market for Regulatory T-cell Therapies, Till 2035
- 9.3.2. Allogeneic Cell Therapy Market: Distribution by Source of Cell, Till 2035
- 9.3.2.1. Allogeneic Cell Therapy Market for Bone Marrow / Matrix-derived Therapies, Till 2035
- 9.3.2.2. Allogeneic Cell Therapy Market for Adipose Tissue-derived Therapies, Till 2035
- 9.3.2.3. Allogeneic Cell Therapy Market for Peripheral Blood-derived Therapies, Till 2035
- 9.3.2.4. Allogeneic Cell Therapy Market for Umbilical Cord-derived Therapies, Till 2035
- 9.3.2.5. Allogeneic Cell Therapy Market for Placenta-derived Therapies, Till 2035
- 9.3.2.6. Allogeneic Cell Therapy Market for Others, Till 2035
- 9.3.3. Allogeneic Cell Therapy Market: Distribution by Target Indication, Till 2035
- 9.3.3.1. Allogeneic Cell Therapy Market for Infectious Disease, Till 2035
- 9.3.3.2. Allogeneic Cell Therapy Market for Chronic Heart Failure, Till 2035
- 9.3.3.3. Allogeneic Cell Therapy Market for Crohn's Disease Till 2035
- 9.3.3.4. Allogeneic Cell Therapy Market for Ischemic Stroke, Till 2035
- 9.3.3.5. Allogeneic Cell Therapy Market for Hematological Cancer, Till 2035
- 9.3.3.6. Allogeneic Cell Therapy Market for Bone Diseases Till 2035
- 9.3.3.7. Allogeneic Cell Therapy Market for Critical Limb Ischemia, Till 2035
- 9.3.3.8. Allogeneic Cell Therapy Market for Graft versus Host Disease, Till 2035
- 9.3.3.9. Allogeneic Cell Therapy Market for Duchenne Muscular Dystrophy, Till 2035
- 9.3.3.10. Allogeneic Cell Therapy Market for Epidermolysis Bullosa, Till 2035
- 9.3.3.11. Allogeneic Cell Therapy Market for Diabetic Foot Ulcer, Till 2035
- 9.3.3.12. Allogeneic Cell Therapy Market for Others, Till 2035
- 9.3.4. Allogeneic Cell Therapy Market: Distribution by Therapeutic Area, Till 2035
- 9.3.4.1. Allogeneic Cell Therapy Market for Post-Transplant Infections, Till 2035
- 9.3.4.2. Allogeneic Cell Therapy Market for Cardiovascular Disorders, Till 2035
- 9.3.4.3. Allogeneic Cell Therapy Market for Autoimmune / Inflammatory Disorders, Till 2035
- 9.3.4.4. Allogeneic Cell Therapy Market for Cerebrovascular Disorders, Till 2035
- 9.3.4.5. Allogeneic Cell Therapy Market for Oncological Disorders, Till 2035
- 9.3.4.6. Allogeneic Cell Therapy Market for Musculoskeletal Disorders, Till 2035
- 9.3.4.7. Allogeneic Cell Therapy Market for Neurological Disorders, Till 2035
- 9.3.4.8. Allogeneic Cell Therapy Market for Genetic Disorders, Till 2035
- 9.3.4.9. Allogeneic Cell Therapy Market for Others, Till 2035
- 9.3.5. Allogeneic Cell Therapy Market: Distribution by Key Geographical Regions, Till 2035
- 9.3.5.1. Allogeneic Cell Therapy Market in North America, Till 2035
- 9.3.5.1.1. Allogeneic Cell Therapy Market in the US, Till 2035
- 9.3.5.1.2. Allogeneic Cell Therapy Market in Canada, Till 2035
- 9.3.5.2. Allogeneic Cell Therapy Market in Europe, Till 2035
- 9.3.5.2.1. Allogeneic Cell Therapy Market in the UK, Till 2035
- 9.3.5.2.2. Allogeneic Cell Therapy Market in Germany, Till 2035
- 9.3.5.2.3. Allogeneic Cell Therapy Market in France, Till 2035
- 9.3.5.2.4. Allogeneic Cell Therapy Market in Italy, Till 2035
- 9.3.5.2.5. Allogeneic Cell Therapy Market in Spain, Till 2035
- 9.3.5.2.6. Allogeneic Cell Therapy Market in Rest of Europe, Till 2035
- 9.3.5.3. Allogeneic Cell Therapy Market in Asia-Pacific and Rest of the World, Till 2035
- 9.3.5.3.1. Allogeneic Cell Therapy Market in India, Till 2035
- 9.3.5.3.2. Allogeneic Cell Therapy Market in Australia, Till 2035
- 9.3.5.3.3. Allogeneic Cell Therapy Market in Taiwan, Till 2035
- 9.3.5.3.4. Allogeneic Cell Therapy Market in Japan, Till 2035
- 9.3.5.3.5. Allogeneic Cell Therapy Market in Korea, Till 2035
- 9.3.5.3.6. Allogeneic Cell Therapy Market in Malaysia, Till 2035
- 9.3.5.3.7. Allogeneic Cell Therapy Market in Israel, Till 2035
- 9.3.5.1. Allogeneic Cell Therapy Market in North America, Till 2035
- 9.3.1. Allogeneic Cell Therapy Market: Distribution by Type of Cell Therapy, Till 2035
- 9.4. Allogeneic Cell Therapy Market: Product-wise Sales Forecast, Till 2035
- 9.4.1. Revascor / MPC-150-IM / Rexlemestrocel-L
- 9.4.1.1. Sales Forecast (USD Million)
- 9.4.1.2. Net Present Value
- 9.4.1.3. Value Creation Analysis
- 9.4.2. Alofisel / Darvadstrocel / Cx601
- 9.4.2.1. Sales Forecast (USD Million)
- 9.4.2.2. Net Present Value
- 9.4.2.3. Value Creation Analysis
- 9.4.3. MultiStem(R) (Athersys)
- 9.4.3.1. Sales Forecast (USD Million)
- 9.4.3.2. Net Present Value
- 9.4.3.3. Value Creation Analysis
- 9.4.4. Tab-cel(R) / tabelecleucel / ATA129
- 9.4.4.1. Sales Forecast (USD Million)
- 9.4.4.2. Net Present Value
- 9.4.4.3. Value Creation Analysis
- 9.4.5. MDR-101
- 9.4.5.1. Sales Forecast (USD Million)
- 9.4.5.2. Net Present Value
- 9.4.5.3. Value Creation Analysis
- 9.4.6. PLX-PAD
- 9.4.6.1. Sales Forecast (USD Million)
- 9.4.6.2. Net Present Value
- 9.4.6.3. Value Creation Analysis
- 9.4.7. OMISIRGE / Omidubicel-onlv
- 9.4.7.1. Sales Forecast (USD Million)
- 9.4.7.2. Net Present Value
- 9.4.7.3. Value Creation Analysis
- 9.4.8. Orca-T
- 9.4.8.1. Sales Forecast (USD Million)
- 9.4.8.2. Net Present Value
- 9.4.8.3. Value Creation Analysis
- 9.4.9. Ryoncil
- 9.4.9.1. Sales Forecast (USD Million)
- 9.4.9.2. Net Present Value
- 9.4.9.3. Value Creation Analysis
- 9.4.10. Stempeucel(R)
- 9.4.10.1. Sales Forecast (USD Million)
- 9.4.10.2. Net Present Value
- 9.4.10.3. Value Creation Analysis
- 9.4.11. Viralym-M / ALVR105 / Posoleucel
- 9.4.11.1. Sales Forecast (USD Million)
- 9.4.11.2. Net Present Value
- 9.4.11.3. Value Creation Analysis
- 9.4.12. Trinity Evolution(R)
- 9.4.12.1. Sales Forecast (USD Million)
- 9.4.12.2. Net Present Value
- 9.4.12.3. Value Creation Analysis
- 9.4.13. CAP-1002
- 9.4.13.1. Sales Forecast (USD Million)
- 9.4.13.2. Net Present Value
- 9.4.13.3. Value Creation Analysis
- 9.4.14. Osteocel(R) Plus
- 9.4.14.1. Sales Forecast (USD Million)
- 9.4.14.2. Net Present Value
- 9.4.14.3. Value Creation Analysis
- 9.4.15. MPC-06-ID / Rexlemestrocel-L
- 9.4.15.1. Sales Forecast (USD Million)
- 9.4.15.2. Net Present Value
- 9.4.15.3. Value Creation Analysis
- 9.4.16. AB-205 / E-CEL cells
- 9.4.16.1. Sales Forecast (USD Million)
- 9.4.16.2. Net Present Value
- 9.4.16.3. Value Creation Analysis
- 9.4.17. allo-APZ2-OTS
- 9.4.17.1. Sales Forecast (USD Million)
- 9.4.17.2. Net Present Value
- 9.4.17.3. Value Creation Analysis
- 9.4.18. Trinity ELITE
- 9.4.18.1. Sales Forecast (USD Million)
- 9.4.18.2. Net Present Value
- 9.4.18.3. Value Creation Analysis
- 9.4.19. CYP-004
- 9.4.19.1. Sales Forecast (USD Million)
- 9.4.19.2. Net Present Value
- 9.4.19.3. Value Creation Analysis
- 9.4.20. Cytovir CMV T-cells
- 9.4.20.1. Sales Forecast (USD Million)
- 9.4.20.2. Net Present Value
- 9.4.20.3. Value Creation Analysis
- 9.4.21. CARTISTEM(R)
- 9.4.21.1. Sales Forecast (USD Million)
- 9.4.21.2. Net Present Value
- 9.4.21.3. Value Creation Analysis
- 9.4.22. Grafix(R)
- 9.4.22.1. Sales Forecast (USD Million)
- 9.4.22.2. Net Present Value
- 9.4.22.3. Value Creation Analysis
- 9.4.23. ELIXCYTE
- 9.4.23.1. Sales Forecast (USD Million)
- 9.4.23.2. Net Present Value
- 9.4.23.3. Value Creation Analysis
- 9.4.24. TEMCELL(R) HS
- 9.4.24.1. Sales Forecast (USD Million)
- 9.4.24.2. Net Present Value
- 9.4.24.3. Value Creation Analysis
- 9.4.25. ALLO-ASC-DFU
- 9.4.25.1. Sales Forecast (USD Million)
- 9.4.25.2. Net Present Value
- 9.4.25.3. Value Creation Analysis
- 9.4.1. Revascor / MPC-150-IM / Rexlemestrocel-L
10. EXECUTIVE INSIGHTS
- 10.1. Chapter Overview
- 10.2. Glycostem Therapeutics
- 10.2.1. Company Snapshot
- 10.2.2. Interview Transcript: Troels Jordansen (Chief Executive Officer)
- 10.3. Mesoblast
- 10.3.1. Company Snapshot
- 10.3.2. Interview Transcript: Eric Rose (Chief Medical Officer)
- 10.4. Triumvira Immunologics
- 10.4.1. Company Snapshot
- 10.4.2. Interview Transcript: Andreas Bader (Chief Scientific Officer)
- 10.5. Celyad Oncology
- 10.5.1. Company Snapshot
- 10.5.2. Interview Transcript: Vincent Brichard (Vice President)













