
|
시장보고서
상품코드
1762522
안티센스 올리고뉴클레오티드 시장 : 업계 동향과 세계 예측 - 안티센스 분자 유형별, ASO 세대 유형별, 표적 적응 질환별, 투여 경로별, 치료 유형별, 지역별Antisense Oligonucleotides Market: Industry Trends and Global Forecasts - Distribution by Type of Antisense Molecule, Type of ASO Generation, Target Disease Indication, Route of Administration, Type of Therapy, and Geography |
||||||
안티센스 올리고뉴클레오티드 시장 : 개요
올해 안티센스 올리고뉴클레오티드 시장 규모는 25억 달러로 예측 기간 중 15%의 연평균 복합 성장률(CAGR)로 확대될 것으로 예측됩니다.
이 시장 세분화에서는 시장 규모와 기회를 다음과 같은 매개 변수로 구분합니다.
안티센스 분자 유형
- DNA 분자
- RNA 분자
ASO 세대 유형
- 1세대 제품
- 2세대 제품
- 3세대 제품
표적 적응증 질환
- 근위축성측색경화증
- 뒤쉔형 근이영양증
- 가족성 카이로미크론혈증 증후군
- 가족성 부분적 저장소 이영양증
- 유전성 트랜스시레틴(hATTR) 아밀로이드증
- 헌팅턴병
- 리베르 선천성 백내장
- 척수성 근위축증
투여 경로
- 척수강내 투여
- 정맥 투여
- 유리체강내 치료
- 피하요법
- 개구부내 치료
치료 유형
- 병용요법
- 단제 치료
지역
- 북미
- 유럽
- 아시아태평양
- 기타 지역
안티센스 올리고뉴클레오티드 시장 : 성장 및 동향
올리고뉴클레오티드는 15-20개의 뉴클레오티드 잔기로 구성된 짧은 단일 가닥 DNA 또는 RNA 분자입니다. 현대 바이오의약품에서 이러한 올리고뉴클레오티드의 용도는 유전학 검사, 기초 생체 분자 연구, 법의학 분석 등 광범위하게 사용되고 있습니다. 안티센스 올리고뉴클레오티드는 다양한 올리고뉴클레오티드의 일종으로, 표적 mRNA에 특이적으로 결합하는 짧은 단일 가닥의 RNA/DNA 분자로, 다양한 메커니즘을 통해 단백질의 발현을 변화시키는 능력을 가지고 있습니다.
안티센스 치료제는 단백질 생산을 손상시키고 인간 유전체의 특정 표적 유전자의 기능을 억제하는 가장 유망한 약물 중 하나로 여겨지고 있습니다. 현재 이 메커니즘은 종양성 질환, 유전성 질환, 간질환, 호흡기 질환, 감염성 질환 등 다양한 질환의 치료를 위해 다양한 단계의 임상시험에서 연구되고 있는 많은 치료제의 기초가 되고 있습니다. 실제로 최근 올리고뉴클레오티드 의약품 개발자들은 코로나바이러스(COVID-19)에 대한 올리고뉴클레오티드 개입의 타당성을 연구하고 있습니다. 안티센스 올리고뉴클레오티드 시장의 기술 혁신과 개발 속도를 고려할 때, 안티센스 올리고뉴클레오티드는 향후 주요 치료 수단이 될 것으로 예측됩니다.
안티센스 올리고뉴클레오티드 시장 : 주요 인사이트
이 보고서는 안티센스 올리고뉴클레오티드 시장의 현황을 조사하고 잠재적인 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
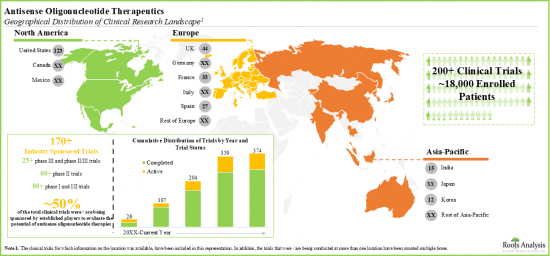
- 안티센스 올리고뉴클레오티드의 잠재적 치료 효과를 평가하기 위해 현재 전 세계 약 30개 기업이 다양한 적응증에 대한 치료를 위해 노력하고 있습니다.
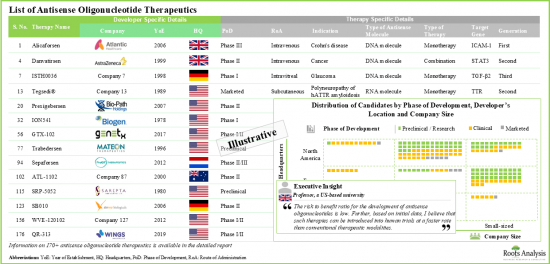
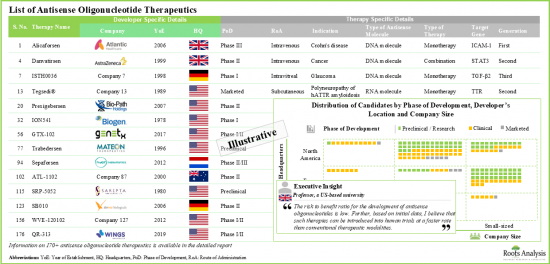
- 파이프라인에는 개발 단계가 다른 170개 이상의 치료제 후보물질이 있으며, 단독 또는 다른 치료제와의 병용요법으로 평가되고 있습니다.
- 승인된 치료제 및 후기 단계 후보물질의 대부분은 유전질환, 신경질환, 종양질환의 치료를 목적으로 하고 있습니다.
- 안티센스 올리고뉴클레오티드의 이점을 고려할 때, 이러한 개입은 주로 단일 요법으로 평가되고 있습니다. 단일 요법으로 연구되고 있는 후기 단계의 약물로는 Tofersen과 Pelacarsen이 있습니다.
- 약 70개의 항센스 올리고뉴클레오티드 기반 치료 후보를 평가하는 임상시험 기관에 18,000명 이상의 환자가 등록되어 있습니다.
- 대부분의 안티센스 올리고뉴클레오티드 치료제는 피하투여용으로 설계되어 있지만, 다양한 약물전달 시스템을 통해 환자가 스스로 투여할 수도 있습니다.
- 이 분야의 연구 활동을 지원하기 위해 여러 기관에서 재정적 지원을 하고 있습니다. 현재, 주로 신경질환의 치료제 연구에 중점을 두고 있습니다.
- 이 분야의 이해관계자(미국)에게 수여된 보조금 수는 지난 수년간 지속적으로 증가하며 왔으며, 총액의 70% 이상이 연구 프로젝트에 수여되었습니다.
- 이 분야에는 NIH의 다양한 관리기관이 참여하고 있는데, 그 중에서도 NINDS, NHLBI, NCI의 참여가 상대적으로 두드러집니다.
- 이 분야에 대한 관심 증가는 다양한 이해관계자들이 다양한 용도에서 맺은 제휴의 수에서도 알 수 있습니다.
- 20개에 가까운 분자가 개발 후기 단계에 있으므로 기업은 주로 제품 개발 및 상용화를 목적으로 공동연구를 진행하고 있습니다.
- 기존 기업이나 신규 진출기업 모두 과거에 전략적 파트너십을 맺은 적이 있으며, 이러한 계약은 주로 유전성 질환과 신경질환에 대한 계약이 주를 이루고 있습니다.
- 시판된 치료제 및 후기 단계 치료제 판매에 따른 매출 측면에서 향후 기회는 다양한 질병 영역, 분자 유형 및 주요 지역적 지역에 잘 분산될 것으로 예측됩니다.
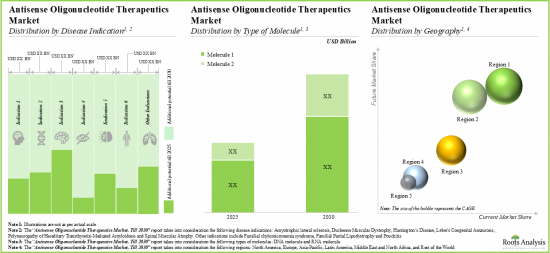
- 이 시장은 향후 10년간 안정적으로 성장할 가능성이 높으며, 그 기회는 다양한 세대, 투여 경로, 다양한 유형의 치료제에 분산될 것으로 보입니다.
안티센스 올리고뉴클레오티드 시장의 참여 기업 예
- Antisense Therapeutics
- Biogen
- Bio-Path Holdings
- Ionis Pharmaceuticals
- ProQR Therapeutics
- Sarepta Therapeutics
- Sterna Biologicals
- Wave Life Sciences
목차
제1장 서문
제2장 개요
제3장 서론
- 챕터 개요
- 올리고뉴클레오티드 및 관련 의약품의 개요
- 올리고뉴클레오티드 유형
- 올리고뉴클레오티드 치료제
- RNA 간섭 치료제
- 안티센스 올리고뉴클레오티드 치료제
- 향후 전망
제4장 안티센스 올리고뉴클레오티드 치료제 : 시장 구도
- 챕터 개요
- 안티센스 올리고뉴클레오티드 치료제 : 파이프라인 리뷰
- 안티센스 분자 유형별 분석
- ASO 세대별 분석
- 개발 단계별 분석
- 표적 유전자별 해석
- 표적 적응 질환별 분석
- 치료 영역별 분석
- 투여 경로별 분석
- 치료법별 분석
- 안티센스 올리고뉴클레오티드 치료제 : 개발자 리스트
- 설립 연별 분석
- 기업 규모별 분석
제4장 본사 소재지별 분석
제5장 기업 개요
- 챕터 개요
- Antisense Therapeutics
- Biogen
- Bio-Path Holdings
- Ionis Pharmaceuticals
- ProQR Therapeutics
- Sarepta Therapeutics
- Sterna Biologicals
- Wave Life Sciences
제6장 임상시험 분석
- 챕터 개요
- 범위와 조사 방법
- 안티센스 올리고뉴클레오티드 치료제 : 임상시험 분석
제7장 학술 보조금 분석
- 챕터 개요
- 범위와 조사 방법
- 안티센스 올리고뉴클레오티드 치료제 : 학술 보조금 분석
제8장 파트너십과 협업
- 챕터 개요
- 파트너십 모델
- 안티센스 올리고뉴클레오티드 치료제 : 제휴·협업 리스트
제9장 시장 예측과 기회 분석
- 챕터 개요
- 예측 조사 방법과 주요 전제조건
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장 : 개별 제품 판매 예측
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장 : 안티센스 분자 유형별
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장 : ASO 세대별
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장 : 표적 적응 질환별
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장 : 투여 경로별
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 치료제 시장 : 치료 유형별
- 2035년까지의 세계 안티센스 올리고뉴클레오티드 시장 : 지역별
제10장 사례 연구 : 올리고뉴클레오티드 제조업체와 정제 서비스
- 챕터 개요
- 조사 및 진단 용도를 전문으로 다루는 올리고뉴클레오티드 제조업체 리스트
- 치료 용도를 전문으로 다루는 올리고뉴클레오티드 제조업체 리스트
제11장 결론
제12장 부록 1 : 표형식 데이터
제13장 부록 2 : 기업·단체 리스트
KSA 25.07.10ANTISENSE OLIGONUCLEOTIDES MARKET: OVERVIEW
As per Roots Analysis, the global antisense oligonucleotides market valued at USD 2.5 billion in the current year is anticipated to grow at a lucrative CAGR of 15% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Antisense Molecule
- DNA Molecules
- RNA Molecules
Type of ASO Generation
- First-Generation Products
- Second-Generation Products
- Third-Generation Products
Target Disease Indication
- Amyotrophic Lateral Sclerosis
- Duchenne Muscular Dystrophy
- Familial Chylomicronemia Syndrome
- Familial Partial Lipodystrophy
- Hereditary Transthyretin-Mediated (hATTR) Amyloidosis
- Huntington's Disease
- Leber's Congenital Amaurosis
- Spinal Muscular Atrophy
Route of Administration
- Intrathecal Therapies
- Intravenous Therapies
- Intravitreal Therapies
- Subcutaneous Therapies
- Intraorifice Therapies
Type of Therapy
- Combination Therapies
- Monotherapies
Geography
- North America
- Europe
- Asia-Pacific
- Rest of the World
ANTISENSE OLIGONUCLEOTIDES MARKET: GROWTH AND TRENDS
Oligonucleotides are short single stranded DNA or RNA molecules, that comprise 15-20 nucleotide residues. In modern biopharmaceuticals, the applications of these oligonucleotides are vast, including (but not limited to) genetic testing, fundamental biomolecular research, and forensic analysis. Antisense oligonucleotides, a diverse class of oligonucleotides are short, single-stranded RNA / DNA molecules specifically binding to the target mRNA and have the ability to modify protein expression through a variety of mechanisms.
Antisense therapeutics are considered to be one of the most promising agents for impairing protein production and blocking the function of the specific target gene of interest in the human genome. Presently, this mechanism forms the basis for many therapeutics being investigated in different stages of clinical trials for treatment of a variety of disorders, including oncological disorders, genetic diseases, hepatic diseases, respiratory disorders and infectious diseases. In fact, in the recent past, the oligonucleotide drug developers had also investigated the relevance of these interventions against the Coronavirus (COVID-19). Given the pace of innovation and developments in the antisense oligonucleotides market, we can expect antisense oligonucleotides to become a major therapeutic modality in the foreseen future.
ANTISENSE OLIGONUCLEOTIDES MARKET: KEY INSIGHTS
The report delves into the current state of the antisense oligonucleotides market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Around 30 players from are presently engaged in evaluating the potential therapeutic benefits of antisense oligonucleotides for the treatment of a wide range of disease indications, worldwide.
- The pipeline features 170+ candidate therapies in different stages of development, being evaluated either as monotherapies or in combination with other interventions; most of these products are administered parenterally.
- Majority of the approved therapies and late-stage candidates are intended for the treatment of genetic disorders, neurological disorders and oncological disorders.
- Given the advantages of antisense oligonucleotides, these interventions are primarily evaluated as monotherapy. Late-stage drugs being investigated as monotherapy include Tofersen and Pelacarsen.
- Over 18,000 patients have been enrolled in clinical trial sites evaluating close to 70 antisense oligonucleotide-based therapy candidates.
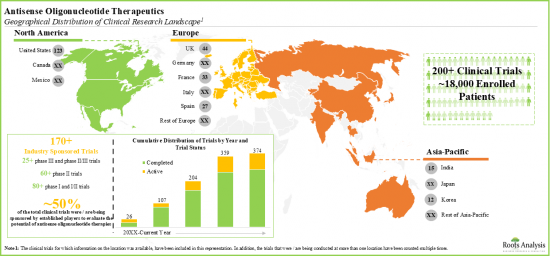
- Majority of the antisense oligonucleotide therapeutics are designed for subcutaneous administration; these can be self-administered by the patients using different drug delivery systems.
- Several organizations have extended financial support to aid research efforts in this domain; currently, the focus, in terms of funds disbursed, is primarily in support of investigations of drugs for treating neurological conditions.
- The number of grants awarded to stakeholders in this domain (in the US) has continuously increased in the past few years; more than 70% of the total amount was awarded for research projects.
- The field has witnessed the involvement of various administering institutes of the NIH; of all the institutes, participation of the NINDS, NHLBI, and NCI has been relatively more prominent.
- The rising interest in this field is reflected in the number of partnerships inked by the various stakeholders across different application areas.
- Given that nearly 20 molecules are in the late stages of development, companies have primarily collaborated for product development and commercialization purposes.
- Both established players and the new entrants have forged strategic partnerships in the recent past; these deals have primarily been inked for genetic and neurological disorders.
- The future opportunity, in terms of revenues from the sales of marketed and late-stage therapies, is anticipated to be well distributed across different disease areas, types of molecules and key geographical regions.
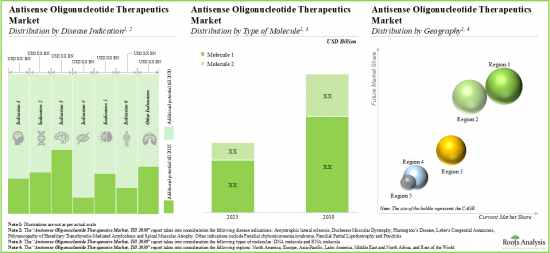
- The market is likely to witness steady growth over the coming decade; the opportunity will be dispersed across different generations, routes of administration and various types of therapies.
Example Players in the Antisense Oligonucleotides Market
- Antisense Therapeutics
- Biogen
- Bio-Path Holdings
- Ionis Pharmaceuticals
- ProQR Therapeutics
- Sarepta Therapeutics
- Sterna Biologicals
- Wave Life Sciences
ANTISENSE OLIGONUCLEOTIDES MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global antisense oligonucleotides market, focusing on key market segments, including [A] type of antisense molecule, [B] type of ASO generation, [C] target disease indication, [D] route of administration, [E] type of therapy and [F] geography.
- Market Landscape: A comprehensive evaluation of antisense oligonucleotide therapeutics, based on several relevant parameters, such as [A] type of antisense molecule, [B] ASO generation, [C] phase of development of lead candidates, [D] target genes, [E] target disease indications, [F] target therapeutic areas, [G] route of administration and [H] type of therapy. Additionally, a comprehensive evaluation of drug developers, based on several relevant parameters, such as [A] year of establishment, [B] company size and [C] location of headquarters.
- Company Profiles: In-depth profiles of antisense oligonucleotide therapeutic developers, focusing on [A] overview of the company, [B] financial information (if available), [C] product portfolio and [D] recent developments and an informed future outlook.
- Clinical Trial Analysis: An insightful analysis of clinical trials related to antisense oligonucleotide therapeutics, based on several parameters, such as [A] trial registration year, [B] trial phase, [C] trial recruitment status, [D] enrolled patient population, [E] study design, leading industry sponsors / collaborators (in terms of number of trials conducted), [F] trial focus, [G] target therapeutic area and [H] target genes.
- Grants Analysis: A comprehensive assessment of grants that have been awarded to research institutes for antisense oligonucleotide therapeutic projects, based on various relevant parameters, such as [A] year of grant award, [B] amount awarded, [C] administering institute center, [D] support period, [E] type of grant application, [F] purpose of grant award, [G] activity code, [H] study section involved, [I] type of recipient organizations and [J] focus area. Additionally, a comprehensive assessment of grants focusing on, [A] geographical distribution of recipient organizations, [B] popular therapeutic areas, [C] popular funding institute centers, [D] prominent program officers and [E] popular recipient organizations.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the antisense oligonucleotide market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] most active players (in terms of number of partnerships signed) and [D] regional analysis.
- Case Study: A detailed discussion on the oligonucleotide CMOs and purification service providers, highlighting information on the [A] year of establishment, [B] company size, [C] scale of operation, [D] location of headquarters and [E] type of purification method used.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview on Oligonucleotides and Affiliated Medical Products
- 3.2.1. Types of Oligonucleotides
- 3.2.1.1. Antisense Oligonucleotides (ASOs)
- 3.2.1.2. Aptamers
- 3.2.1.3. miRNA
- 3.2.1.4. shRNA
- 3.2.1.5. siRNA
- 3.2.1.6. Other Oligonucleotides
- 3.2.1. Types of Oligonucleotides
- 3.3. Oligonucleotide Therapeutics
- 3.3.1. RNA-Interference Therapeutics
- 3.3.1.1. Components of RNA-Interference Therapeutics
- 3.3.1.2. Mechanism of RNA-Interference Therapeutics
- 3.3.2. Antisense Oligonucleotide Therapeutics
- 3.3.2.1. Mechanism of Antisense Oligonucleotide Therapeutics
- 3.3.2.2. Types of Antisense Oligonucleotide Therapeutics
- 3.3.1. RNA-Interference Therapeutics
- 3.4. Future Perspectives
4. ANTISENSE OLIGONUCLEOTIDE THERAPEUTICS: MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Antisense Oligonucleotide Therapeutics: Pipeline Review
- 4.2.1. Analysis by Type of Antisense Molecule
- 4.2.2. Analysis by ASO Generation
- 4.2.3. Analysis by Phase of Development
- 4.2.4. Analysis by Target Genes
- 4.2.5. Analysis by Target Indications
- 4.2.6. Analysis by Therapeutic Areas
- 4.2.7. Analysis by Route of Administration
- 4.2.8. Analysis by Type of Therapy
- 4.3. Antisense Oligonucleotide Therapeutics: List of Developers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
4. 3.3. Analysis by Location of Headquarters
- 4.4. Grid Analysis: Distribution by Phase of Development, Company Size and Location of Headquarters
5. COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Antisense Therapeutics
- 5.2.1. Company Overview
- 5.2.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Biogen
- 5.3.1. Company Overview
- 5.3.2. Financial Information
- 5.3.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.3.4. Recent Developments and Future Outlook
- 5.4. Bio-Path Holdings
- 5.4.1. Company Overview
- 5.4.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Ionis Pharmaceuticals
- 5.5.1. Company Overview
- 5.5.2. Financial Information
- 5.5.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.5.4. Recent Developments and Future Outlook
- 5.6. ProQR Therapeutics
- 5.6.1. Company Overview
- 5.6.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.6.3. Recent Developments and Future Outlook
- 5.7. Sarepta Therapeutics
- 5.7.1. Company Overview
- 5.7.2. Financial Information
- 5.7.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.7.4. Recent Developments and Future Outlook
- 5.8. Sterna Biologicals
- 5.8.1. Company Overview
- 5.8.2. Antisense Oligonucleotide Therapeutics Portfolio
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Wave Life Sciences
- 5.9.1. Company Overview
- 5.9.2. Financial Information
- 5.9.3. Antisense Oligonucleotide Therapeutics Portfolio
- 5.9.4. Recent Developments and Future Outlook
6. CLINICAL TRIAL ANALYSIS
- 6.1. Chapter Overview
- 6.2. Scope and Methodology
- 6.3. Antisense Oligonucleotide Therapeutics: Clinical Trial Analysis
- 6.3.1. Analysis by Trial Registration Year
- 6.3.2. Analysis by Trial Phase
- 6.3.3. Analysis by Trial Recruitment Status
- 6.3.4. Analysis by Trial Registration Year and Number of Patients Enrolled
- 6.3.5. Analysis by Study Design
- 6.3.6. Analysis by Type of Sponsor / Collaborator
- 6.3.7. Leading Players: Analysis by Number of Registered Trials
- 6.3.8. Word Cloud: Key Focus Areas
- 6.3.9. Analysis by Target Therapeutic Area
- 6.3.10. Analysis by Trial Registration Year and Target Gene
- 6.3.11. Popular Indications: Analysis by Number of Registered Trials
- 6.3.12. Popular Interventions: Analysis by Number of Registered Trials
- 6.3.13. Geographical Analysis by Number of Registered Trials
- 6.3.14. Geographical Analysis by Number of Patients Enrolled
7. ACADEMIC GRANTS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Antisense Oligonucleotide Therapeutics: Analysis of Academic Grants
- 7.3.1. Analysis by Year of Grant Award
- 7.3.2. Analysis by Amount Awarded
- 7.3.3. Analysis by Administering Institute Center
- 7.3.4. Analysis by Support Period
- 7.3.5. Analysis by Administering Institute Center and Support Period
- 7.3.6. Analysis by Type of Grant Application
- 7.3.7. Analysis by Purpose of Grant Award
- 7.3.8. Analysis by Activity Code
- 7.3.9. Analysis by Study Section Involved
- 7.3.10. Analysis by Type of Recipient Organization
- 7.3.11. Word Cloud Analysis: Emerging Focus Areas
- 7.3.12. Geographical Distribution of Recipient Organizations
- 7.3.13. Popular Therapeutic Areas: Analysis by Number of Grants
- 7.3.14. Popular NIH Departments: Analysis by Number of Grants
- 7.3.15. Prominent Program Officers: Analysis by Number of Grants
- 7.3.16. Popular Recipient Organizations: Analysis by Number of Grants
8. PARTNERSHIPS AND COLLABORATIONS
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Antisense Oligonucleotide Therapeutics: List of Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type of Partnership and Generation of Antisense Molecule Involved
- 8.3.4. Analysis by Type of Partnership and Target Therapeutic Area
- 8.3.5. Analysis by Year of Partnership and Type of Partner
- 8.3.6. Analysis by Type of Partnership and Type of Partner
- 8.3.7. Most Active Players: Analysis by Number of Partnerships
- 8.3.8. Regional Analysis
- 8.3.8.1. Intercontinental and Intracontinental Agreements
9. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 9.1. Chapter Overview
- 9.2. Forecast Methodology and Key Assumptions
- 9.3. Global Antisense Oligonucleotide Therapeutics Market, Till 2035
- 9.4. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Individual Product Sales Forecasts
- 9.4.1. Alicaforsen (Atlantic Healthcare)
- 9.4.1.1. Target Patient Population
- 9.4.1.2. Sales Forecast
- 9.4.2. Eteplirsen (Sarepta Therapeutics)
- 9.4.2.1. Target Patient Population
- 9.4.2.2. Sales Forecast
- 9.4.3. Golodirsen (Sarepta Therapeutics)
- 9.4.3.1. Target Patient Population
- 9.4.3.2. Sales Forecast
- 9.4.4. Inotersen (Ionis Pharmaceuticals)
- 9.4.4.1. Target Patient Population
- 9.4.4.2. Sales Forecast
- 9.4.5. Sepofarsen (ProQR Therapeutics)
- 9.4.5.1. Target Patient Population
- 9.4.5.2. Sales Forecast
- 9.4.6. Tofersen (Biogen)
- 9.4.6.1. Target Patient Population
- 9.4.6.2. Sales Forecast
- 9.4.7. Tominersen (Roche)
- 9.4.7.1. Target Patient Population
- 9.4.7.2. Sales Forecast
- 9.4.8. Viltolarsen (Nippon Shinyaku)
- 9.4.8.1. Target Patient Population
- 9.4.8.2. Sales Forecast
- 9.4.9. Volanesorsen (Ionis Pharmaceuticals)
- 9.4.9.1. Target Patient Population
- 9.4.9.2. Sales Forecast
- 9.4.1. Alicaforsen (Atlantic Healthcare)
- 9.5. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Antisense Molecule
- 9.5.1. Global Antisense Oligonucleotide Therapeutics Market for RNA Molecules, Till 2035
- 9.5.2. Global Antisense Oligonucleotide Therapeutics Market for DNA Molecules, Till 2035
- 9.6. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by ASO Generation
- 9.6.1. Global Antisense Oligonucleotide Therapeutics Market for First-generation Products, Till 2035
- 9.6.2. Global Antisense Oligonucleotide Therapeutics Market for Second-generation Products, Till 2035
- 9.6.3. Global Antisense Oligonucleotide Therapeutics Market for Third-generation Products, Till 2035
- 9.7. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Target Disease Indication
- 9.7.1. Global Antisense Oligonucleotide Therapeutics Market for Amyotrophic Lateral Sclerosis, Till 2035
- 9.7.2. Global Antisense Oligonucleotide Therapeutics Market for Duchenne Muscular Dystrophy, Till 2035
- 9.7.3. Global Antisense Oligonucleotide Therapeutics Market for Familial Chylomicronemia Syndrome, Till 2035
- 9.7.4. Global Antisense Oligonucleotide Therapeutics Market for Familial Partial Lipodystrophy, Till 2035
- 9.7.5. Global Antisense Oligonucleotide Therapeutics Market for Hereditary Transthyretin-Mediated (hATTR) Amyloidosis, Till 2035
- 9.7.6. Global Antisense Oligonucleotide Therapeutics Market for Huntington's Disease, Till 2035
- 9.7.7. Global Antisense Oligonucleotide Therapeutics Market for Leber's Congenital Amaurosis, Till 2035
- 9.7.8. Global Antisense Oligonucleotide Therapeutics Market for Pouchitis, Till 2035
- 9.7.9. Global Antisense Oligonucleotide Therapeutics Market for Spinal Muscular Atrophy, Till 2035
- 9.8. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Route of Administration
- 9.8.1. Global Antisense Oligonucleotide Therapeutics Market for Intrathecal Therapies, Till 2035
- 9.8.2. Global Antisense Oligonucleotide Therapeutics Market for Intravenous Therapies, Till 2035
- 9.8.3. Global Antisense Oligonucleotide Therapeutics Market for Intravitreal Therapies, Till 2035
- 9.8.4. Global Antisense Oligonucleotide Therapeutics Market for Subcutaneous Therapies, Till 2035
- 9.8.5. Global Antisense Oligonucleotide Therapeutics Market for Intraorifice Therapies, Till 2035
- 9.9. Global Antisense Oligonucleotide Therapeutics Market, Till 2035: Distribution by Type of Therapy
- 9.9.1. Global Antisense Oligonucleotide Therapeutics Market for Combination Therapies Till 2035
- 9.9.2. Global Antisense Oligonucleotide Therapeutics Market for Monotherapies, Till 2035
- 9.10. Global Antisense Oligonucleotide Market, Till 2035: Geographical Distribution
- 9.10.1. Antisense Oligonucleotide Market in the US, Till 2035
- 9.10.2. Antisense Oligonucleotide Market in Canada, Till 2035
- 9.10.3. Antisense Oligonucleotide Market in the UK, Till 2035
- 9.10.4. Antisense Oligonucleotide Market in Germany, Till 2035
- 9.10.5. Antisense Oligonucleotide Market in France, Till 2035
- 9.10.6. Antisense Oligonucleotide Market in Italy, Till 2035
- 9.10.7. Antisense Oligonucleotide Market in Spain, Till 2035
- 9.10.8. Antisense Oligonucleotide Market in Australia, Till 2035
- 9.10.9. Antisense Oligonucleotide Market in Japan, Till 2035
- 9.10.10. Antisense Oligonucleotide Market in Korea, Till 2035
- 9.10.11. Antisense Oligonucleotide Market in Brazil, Till 2035
- 9.10.12. Antisense Oligonucleotide Market in Israel, Till 2035
10. CASE STUDY: OLIGONUCLEOTIDE MANUFACTURERS AND PURIFICATION SERVICES
- 10.1. Chapter Overview
- 10.2. List of Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications
- 10.2.1. Analysis by Year of Establishment
- 10.2.2. Analysis by Company Size
- 10.2.3. Analysis by Scale of Operation
- 10.2.4. Analysis by Location of Headquarters
- 10.2.5. Analysis by Type of Purification Method Used
- 10.3. List of Oligonucleotide Manufacturers Focused on Therapeutic Applications
- 10.3.1. Analysis by Year of Establishment
- 10.3.2. Analysis by Company Size
- 10.3.3. Analysis by Scale of Operation
- 10.3.4. Analysis by Location of Headquarters
- 10.3.5. Analysis by Type of Purification Method Used













