
|
시장보고서
상품코드
1891248
생물제제 임상시험 수탁기관(CRO) 시장 : 생물제제 유형별, 사업 규모별, 치료 영역별, 지역별 업계 동향과 예측Biologics Contract Research Organization Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Type of Biologic, Scale of Operation, Therapeutic Area, and Geography |
||||||
생물제제 R&D 수탁기관(CRO) 시장 - 개요
세계의 생물제제 R&D 수탁기관(CRO) 시장 규모는 올해 360억 달러로, 예측 기간 동안 13%라는 높은 CAGR로 성장해 2035년까지 1,260억 달러에 이를 것으로 전망됩니다.
시장 규모 및 기회 분석은 다음 매개변수에 따라 세분화됩니다.
생물제제 유형
- 백신
- 세포 치료
- 유전자 치료
- 항체
- 재조합 단백질 및 펩티드
- 기타
사업 규모
- 임상 업무
- 전임상 업무
치료 영역
- 종양 질환
- 심혈관 질환
- 염증성 질환
- 신경 질환
- 기타
지역
- 북미
- 유럽
- 아시아태평양
- 라틴아메리카
- 중동 및 북아프리카
- 기타 지역
생물제제 R&D 수탁기관(CRO) 시장 - 성장과 동향
생물제제는 제약 업계에서 가장 빠르게 확대되는 분야 중 하나입니다.
이는 효율적이고 개인화된 약리학적 솔루션에 대한 수요에 힘입은 이 분야의 빠른 발전과 관련이 있습니다. 단일클론항체, 유전자 치료, 세포 치료를 포함한 생물제제 수요 증가는 암, 자가면역 질환, 신경 질환 등의 만성적이고 복잡한 질환에 성공적으로 대처하면서 적극 추진되고 있습니다. 이 수요는 확대되는 생물제제의 파이프라인과 승인율 증가에 의해 더욱 강해지고 있습니다. 그러나 생물제제의 개발과 임상 평가는 전문 지식과 최첨단 바이오프로세스 기술을 필요로 하는 매우 복잡하고 비용이 많이 드는 프로세스입니다. 따라서, 많은 생물제제 기업은 신약 개발 및 임상 연구 활동을 외부에 위탁합니다.
현재 생물제제 기업은 개발 프로세스를 외부 서비스 제공업체에 위탁하고 자사에서 이러한 생물제제의 연구 개발을 감독하는 비즈니스 모델을 채택하고 있습니다. 생물제제 CRO(임상시험 수탁기관) 업계는 혁신적인 의약품 및 질병에 관한 연구 증가에 힘입어 성장을 이루고 있습니다. 생물제제의 연구 개발은 노동 집약적이며, 제약 기업은 첨단 기술 지식과 전문 지식이 필요하므로 의약품의 총 비용이 증가합니다. 게다가, 엄격한 규제 기준과 승인 과정은 의약품 승인을 지연시켜 시장에서 의약품 공급 부족 현상을 초래할 수 있습니다.
이러한 복잡한 프로세스를 간소화하고 관련 과제를 해결하기 위해 주요 제약 기업은 생물제제의 연구 개발 업무를 전문 CRO(임상시험 수탁기관)에 단계적으로 위탁하고 있습니다. 이 전략적 외부 위탁은 외부 지식의 활용, 신약 개발 및 임상시험의 신속화를 가능하게 하고, 궁극적으로 혁신적 생물제제 시장 투입을 효율적으로 실현할 수 있습니다. 게다가 AI의 통합으로 신약 개발의 가속, 임상시험(환자 모집 등)의 효율화, 제조 수율의 향상이 기대됩니다. 바이오프로세스 자동화와 분산 및 가상 임상시험의 도입에는 수많은 기회가 존재하며, 이들은 비용 절감과 데이터 품질의 향상으로 이어집니다. 따라서, 증가하는 연구 개발 요구에 부응하기 위해 생물제제 CRO 시장은 꾸준한 성장이 예상되고 있습니다.
생물제제 CRO 시장 - 주요 인사이트
이 보고서는 생물제제 CRO 시장의 현재 상태를 상세하게 분석하고 업계 내 잠재적 성장 기회를 확인합니다. 주요 조사 결과는 다음과 같습니다.
- 현재 160개 기업이 다양한 유형의 생물제제 제품에 대한 수탁 연구 서비스 및 임상시험 지원을 제공하는 데 필요한 능력을 보유하고 있습니다.
- 전체 사업자의 약 50%는 임상 서비스만을 제공하고 있으며, 그 중 12%의 CRO가 생물제제의 임상 연구와 관련된 전체 서비스를 제공합니다.

- 현재 시장 상황은 분산화가 진행되고 있으며, 확립된 기업과 전문 서비스 제공업체가 혼재하여 전임상 및 임상 연구를 지원하는 광범위한 포트폴리오를 보유하고 있습니다.
- 제약 대기업은 현재 내부 연구 개발 업무의 약 45%를 CRO에 위탁하고 있는 것으로 추정됩니다.
- 수탁 연구 서비스 제공업체의 3분의 2 이상은 북미 및 유럽에 본사를 두고 있으며, 대부분은 중소기업입니다.
- 경쟁 우위를 확보하기 위해 각 회사는 기존의 능력을 적극적으로 확대하고, 각각의 제공 서비스를 더욱 강화하여 진화하는 업계 기준과의 적합성을 도모하고 있습니다.

- 현재 8,000건 이상의 제품 후보가 생물제제 개발 기업별로 평가 및 개발 중이며, 생물제제 CRO 시장에 기회를 가져오고 있습니다.
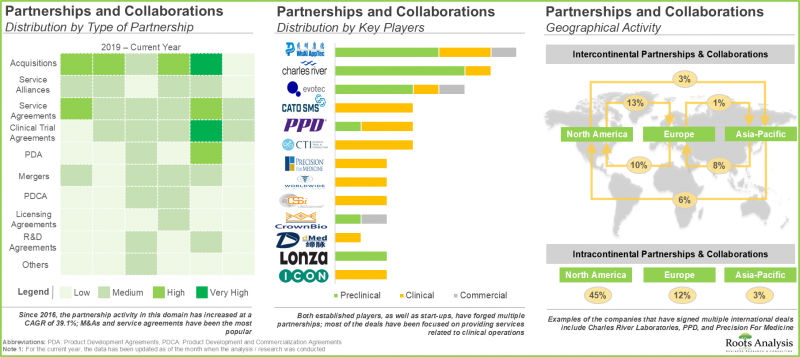
- 이 분야의 이해관계자들의 관심 증가는 최근의 제휴 활동 증가에도 반영되고 있습니다. 2016년 이후 업계 관계자는 스폰서 및 기타 CRO와 여러 계약을 체결했습니다.
- CRO의 약 20%가 임상 및 전임상 수준에서 생물제제에 대한 연구 서비스를 제공합니다.
- 원스톱 서비스에 대한 수요가 증가함에 따라 생물제제 연구와 관련된 능력의 통합을 적극적으로 추진하고 있는 업계 이해관계자는 주로 합병, 인수 및 내부 확충을 통해 그 능력을 통합하고 있습니다.

- 향후 10년간 시장은 CAGR 약 13%로 확대될 것으로 예측되고 있습니다. 이러한 성장 기회는 치료 영역, 사업 규모, 생물제제 유형, 최종 사용자, 지리적 지역에 걸쳐 균등하게 분산될 가능성이 높습니다.
생물제제 R&D 수탁기관(CRO) 시장 - 주요 부문
임상 부문이 생물제제 CRO 시장에서 가장 큰 점유율을 차지할 전망
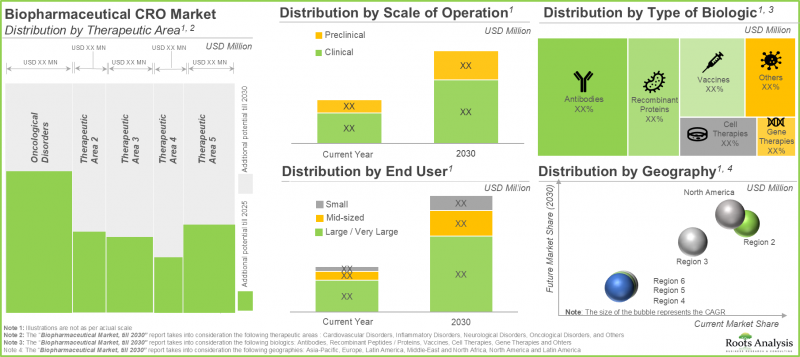
사업 규모의 관점에서 세계의 생물제제 CRO 시장은 신약 개발, 전임상, 임상의 3가지로 구분됩니다. 현재 시장 점유율의 대부분은 임상 규모의 사업이 차지하고 있으며, 이어서 전임상, 신약 개발 부문이 뒤따르고 있습니다.
북미가 생물제제 CRO 시장에서 가장 큰 점유율을 획득할 것으로 예측됩니다.
지역별로는 북미, 유럽, 아시아태평양으로 구분됩니다. 이 중 북미가 시장 점유율의 대부분(50%)을 차지할 것으로 예측되며, 이어 유럽(32%), 아시아태평양(16%)이 이어집니다.
생물제제 CRO 시장의 대표 기업 예
- Biocon
- Covance
- ICON
- Medpace
- Pharmaron
- PPD
- PRA Health Sciences
- Syneos Health
- Vimta Labs
- WuXi AppTec
생물제제 CRO 시장 - 조사 범위
- 시장 규모 및 기회분석 : 본 보고서에서는 (A)생물제제형, (B)사업 규모, (C)치료영역, (D)지역 등 주요 시장부문에 초점을 맞추어 세계의 생물제제 임상시험 수탁기관 시장의 상세한 분석을 제공합니다.
- 시장 상황 : 생물제제 임상시험 수탁기관 시장을 (A)설립연도, (B)기업 규모, (C)본사 소재지, (D)제조 생물제제 유형, (E)사업 규모, (F)제공 서비스 유형(임상 서비스, 비임상 서비스 포함) 등 관련 매개변수에 따라 종합적으로 평가합니다.
- 기업 프로파일 : 생물제제 관련 서비스를 제공하는 CRO(임상시험 수탁기관)의 상세한 프로파일을 제공하며 특히 (A)기업 개요, (B)재무 정보(공개된 경우), (C)서비스 포트폴리오, (D)최근 동향과 미래 전망에 중점을 둡니다.
- 벤치마킹 분석 : 경쟁 우위를 위해 피어 그룹의 기업 능력에 따라 생물제제 임상시험 수탁기관 시장의 진출기업에 대한 종합적인 벤치마킹 분석을 제공합니다.
- 제휴 및 협업 : 생물제제 CRO 시장 내 이해 관계자의 계약 체결 상황을 (A)제휴 연수, (B)제휴 형태, (C)가장 활발한 참가 기업(체결 계약수 기준), (D)사업 규모, (E)지역 등의 복수의 파라미터에 근거해 인사이트가 풍부한 분석을 실시합니다.
- 합병 및 인수 : 본 분야의 합병 및 인수 활동의 상세한 분석을 제공합니다. 관련 파라미터로서 (A)인수연도, (B)협업 형태, (C)지역, (D)가장 활발한 인수기업, (E)소유권변경 매트릭스, (F)주요가치 촉진요인, (G)인수거래배율을 바탕으로 분석을 실시합니다.
- 매력 및 경쟁력 매트릭스 : 2016년 이후 타사를 인수한 기업의 활동 이력을 고려한 종합적인 인수를 대상으로 분석을 실시하며 타업계 참여 기업이 잠재적인 인수 대상을 특정하는 수단을 제공합니다.
- SWOT 분석 : 생물제제 CRO 시장의 진화에 영향을 줄 수 있는 업계 관련 동향, 기회 및 과제를 분석합니다. 하비볼 분석을 포함하여 각 SWOT 파라미터가 업계 역학에 미치는 상대적인 영향을 평가합니다.
목차
제1장 서문
제2장 주요 요약
제3장 소개
제3장 소개
- 개요
- 생물제제의 개요
- 임상시험 수탁기관(CRO)의 개요
- CRO의 진화
- CRO의 분류
- CRO의 제공 서비스
- CRO에의 업무 아웃소싱의 장점
- CRO에의 업무 아웃소싱에 수반하는 리스크
제4장 아웃소싱 가이드
- 개요
- 생물제제 연구의 아웃소싱
- 아웃소싱 모델
- 적절한 아웃소싱 모델 선택
- CRO 파트너 선택
- 의약품 개발 프로세스에서의 CRO의 역할
- 결론
제5장 시장 상황
- 개요
- 생물제제 CRO : 시장 상황
- 전임상 생물제제 CRO
- 임상 생물제제 CRO
제6장 기업 프로파일
- 개요
- Biocon
- Covance
- ICON
- Medpace
- Pharmaron
- PPD
- PRA Health Sciences
- Syneos Health
- Vimta Labs
- WuXi AppTec
제7장 벤치마크 분석
- 개요
- 조사 방법
- 벤치마크 분석 : 피어 그룹
제8장 파트너십 및 협업
- 개요
- 파트너십 모델
- 생물제제 CRO : 최근의 파트너십 및 협업
제9장 합병과 인수
제10장 매력 및 경쟁력 매트릭스
- 개요
- AC 매트릭스 : 개요
- 분석조사방법
- AC 매트릭스 : 북미의 계약 제조 시나리오
- AC 매트릭스 : 유럽의 계약 제조 시나리오
- AC 매트릭스 : 아시아태평양 및 중동의 계약 제조 시나리오
제11장 시장 예측
- 개요
- 예측 조사 방법과 주요 전제조건
- 세계의 생물제제 CRO 서비스 시장
- 북미의 생물제제 CRO 서비스 시장
- 유럽의 생물제제 CRO 서비스 시장
- 아시아태평양의 생물제제 CRO 서비스 시장
- 라틴아메리카의 생물제제 CRO 서비스 시장
- 중동 북미의 생물제제 CRO 서비스 시장
제12장 SWOT 분석
제13장 결론
제14장 조사 기록
제15장 부록 I : 테이블 데이터
제16장 부록 II : 기업 및 조직 목록
CSM 25.12.26Biologics Contract Research Organization Market: Overview
As per Roots Analysis, the global biologics contract research organization market valued at USD 36 billion in the current year and is anticipated to reach USD 126 billion by 2035, growing at a lucrative CAGR of 13% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Biologic
- Vaccines
- Cell Therapy
- Gene Therapy
- Antibodies
- Recombinant Proteins / Peptides
- Others
Scale of Operation
- Clinical Operations
- Preclinical Operations
Therapeutic Area
- Oncological Disorders
- Cardiovascular Disorders
- Inflammatory Disorders
- Neurological Disorders
- Other Therapeutic Areas
Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and North Africa
- Rest of the World
Biologics Contract Research Organization Market: Growth and Trends
Biologics are among the fastest expanding sectors within the pharmaceutical industry. This
can be linked to the swift advancement in this area, fueled by the demand for efficient and tailored pharmacological solutions. The increased need for biologics including monoclonal antibodies, gene therapies, and cell therapies is driven by their success in addressing chronic and complex ailments such as cancer, autoimmune diseases, and neurological disorders. This need is intensified by an expanding biologics pipeline and elevated approval rates. Nonetheless, the development and clinical evaluation of biologics is a very intricate and expensive procedure that requires specialized knowledge and cutting-edge bioprocessing technologies. Consequently, numerous biopharmaceutical firms are opting to delegate their drug discovery and clinical research activities.
At present, biopharmaceutical firms are adopting a business model wherein development processes are contracted to external service providers, while the firm itself oversees the R&D of these biologics. The biologics contract research organization sector is experiencing growth fueled by the rising research on innovative medications and illnesses. R&D of biologics is labor-intensive and pharmaceutical companies need significant technological knowledge and specialized expertise, which increases the overall expense of drugs. Moreover, strict regulatory standards and the approval process can delay the approval of drugs, resulting in a drug supply shortfall in the market.
To simplify these intricate processes and address related challenges, leading pharmaceutical companies are progressively delegating their biologics research and development efforts to specialized contract research organizations (CROs). This strategic outsourcing enables them to utilize external knowledge, speed up drug discovery and clinical trials, and ultimately launch innovative biologics to market more effectively. In addition, the integration of AI can speed up drug discovery, enhance clinical trials (such as patient recruitment), and boost manufacturing yields. The domain presents numerous opportunities in implementing automation for bioprocessing and decentralized / virtual clinical trials, which lower expenses and improve data quality. As a result, the biologics contract research organization market is expected to grow steadily to meet the increasing R&D needs of biopharmaceutical sponsors.
Biologics Contract Research Organization Market: Key Insights
The report delves into the current state of the biologics contract research organization market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, 160 players claim to have the necessary capabilities to offer contract research services and clinical trial support for different types of biopharmaceutical products.
- About 50% of all the players offer only clinical services; of these, 12% CROs provide all the services associated with clinical research of biologics.
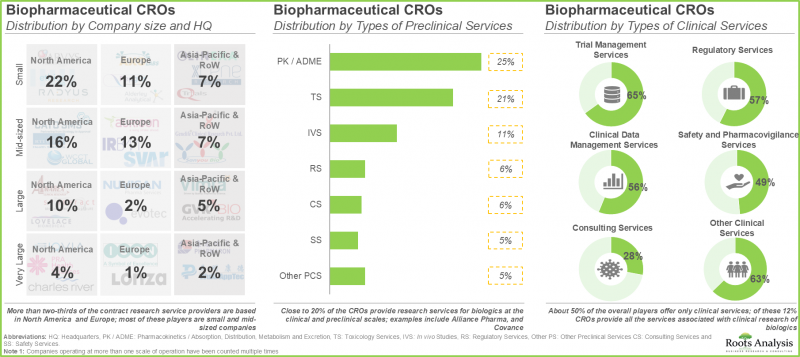
- The current market landscape is fragmented, featuring a mix of well-established players and specialty service providers, having extensive portfolios to support preclinical and clinical research.
- It is estimated that big pharma players presently outsource close to 45% of their internal R&D operations to CROs.
- More than two-thirds of the contract research service providers are based in North America and Europe; most of these players are small and mid-sized companies.
- In order to gain a competitive edge, companies are actively expanding their existing capabilities in order to further augment their respective offerings and also comply with evolving industry benchmarks.
- Over 8,000 product candidates are currently being evaluated / under development biologic drug developers, presenting opportunities in the biologics contract research organization market.
- The growing interest of stakeholders in this field is also reflected in the increase in partnership activity in the recent past; since 2016, industry players have signed multiple deals with sponsor and / or other CROs.
- Close to 20% of the CROs provide research services for biologics at the clinical and preclinical scales.
- With the rising demand for one-stop shops, industry stakeholders are actively consolidating their capabilities related to biopharmaceutical research, mostly through mergers and acquisitions, and internal expansions.
- The market is expected to grow at a CAGR of ~13% in the coming decade; the opportunity is likely to be well distributed across therapeutic areas, scales of operation, types of biologics, end users and geographical regions.
Biologics Contract Research Organization Market: Key Segments
Clinical Segment is Likely to Hold the Highest Share in the Biologics Contract Research Organization Market
In terms of the scale of operation, the global market for biologics contract research organization market is segmented into discovery, preclinical and clinical. Currently, majority share of the share is captured by clinical scale of operation, followed by preclinical and discovery segments.
North America is Anticipated to Capture the Maximum Biologics Contract Research Organization Market Share
In terms of geographical regions, the global market is segmented into North America, Europe and Asia-Pacific. Amongst these, North America is likely to capture majority (50%) of the market share, followed by Europe (32%) and Asia-Pacific (16%).
Example Players in the Biologics Contract Research Organization Market
- Biocon
- Covance
- ICON
- Medpace
- Pharmaron
- PPD
- PRA Health Sciences
- Syneos Health
- Vimta Labs
- WuXi AppTec
Biologics Contract Research Organization Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global biologics contract research organization market, focusing on key market segments, including [A] type of biologic, [B] scale of operation, [C] therapeutic area and [D] geography.
- Market Landscape: A comprehensive evaluation of the biopharmaceutical services market, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] types of biologics manufactured, [E] scale of operation and [F] types of services offered (including clinical services and preclinical services).
- Company Profiles: In-depth profiles of CROs engaged in offering biopharmaceutical related services, focusing on [A] overview of the company, [B] financial information (if available), [C] service portfolio and [D] recent developments and an informed future outlook.
- Benchmarking Analysis: A comprehensive benchmark analysis of players engaged in biologics contract research organization market based on the [A] capabilities of companies within a peer group with an aim to gain a competitive edge.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the biologics contract research organization market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] most active players (in terms of number of partnerships signed), [D] scale of operation and [E] geography.
- Mergers and Acquisitions: An in-depth analysis of the mergers and acquisitions undertaken in this domain, based on relevant parameters, such as [A] year of acquisition, [B] type of collaboration, [C] geography, [D] most active acquirers, [E] ownership change matrix, [F] key value drivers and [G] acquisition deal multiples.
- Attractiveness Competitiveness Matrix: A comprehensive acquisition target analysis, considering the historical trend of the activity of companies that have acquired other firms since 2016, and providing a means for other industry players to identify potential acquisition targets.
- SWOT Analysis: An analysis of industry affiliated trends, opportunities and challenges, which are likely to impact the evolution of biologics contract research organization market; it includes a Harvey ball analysis, assessing the relative impact of each SWOT parameter on industry dynamics.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
3 Introduction
- 3.1. Chapter Overview
- 3.2. Overview of Biologics
- 3.2.1. Types of Biologics
- 3.3. Overview of Contract Research Organizations (CROs)
- 3.4. Evolution of CROs
- 3.5. Classification of CROs
- 3.6. Services Offered by CROs
- 3.7. Advantages of Outsourcing Operations to CROs
- 3.8. Risks Associated with Outsourcing Operations to CROs
4. GUIDE TO OUTSOURCING
- 4.1. Chapter Overview
- 4.2. Outsourcing Biologics Research
- 4.3. Models of Outsourcing
- 4.3.1. Transactional Outsourcing Model
- 4.3.2. Performance / Outcome-based Business Model
- 4.3.3. Staff Augmentation Model
- 4.3.4. Phase-Dependent Outsourcing Model
- 4.4. Selecting an Appropriate Outsourcing Model
- 4.4.1. Hybrid Approach
- 4.5. Selecting a CRO Partner
- 4.5.1. Key Considerations for Outsourcing Biologics-related Operations
- 4.6. Role of CROs in the Drug Development Process
- 4.6.1. Discovery and Preclinical Research
- 4.6.2. Early Clinical Research
- 4.6.3. Clinical Research and Laboratory Services
- 4.7. Conclusion
5. MARKET LANDSCAPE
- 5.1. Chapter Overview
- 5.2. Biopharmaceutical CROs: Overall Market Landscape
- 5.2.1. Analysis by Year of Establishment, Company Size and Location of Headquarters
- 5.2.2. Analysis by Scale of Operation
- 5.3. Preclinical Biopharmaceutical CROs
- 5.3.1. Analysis by Year of Establishment
- 5.3.2. Analysis by Company Size
- 5.3.3. Analysis by Location of Headquarters
- 5.3.4. Analysis by Type of Biologic
- 5.3.5. Analysis by Type of Services Offered
- 5.4. Clinical Biopharmaceutical CROs
- 5.4.1. Analysis by Year of Establishment
- 5.4.2. Analysis by Company Size
- 5.4.3. Analysis by Location of Headquarters
- 5.4.4. Analysis by Type of Biologics
- 5.4.5. Analysis by Type of Services Offered
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Biocon
- 6.2.1. Company Overview
- 6.2.2. Financial Information
- 6.2.3. Services Portfolio
- 6.2.4. Future Outlook
- 6.3. Covance
- 6.3.1. Company Overview
- 6.3.2. Service Portfolio
- 6.3.3. Financial Information
- 6.3.4. Future Outlook
- 6.4. ICON
- 6.4.1. Company Overview
- 6.4.2. Financial Information
- 6.4.3. Service Portfolio
- 6.4.4. Future Outlook
- 6.5. Medpace
- 6.5.1. Company Overview
- 6.5.2. Financial Information
- 6.5.3. Service Portfolio
- 6.5.4. Future Outlook
- 6.6. Pharmaron
- 6.6.1. Company Overview
- 6.6.2. Services Portfolio
- 6.6.3. Future Outlook
- 6.7. PPD
- 6.7.1. Company Overview
- 6.7.2. Service Portfolio
- 6.7.3. Financial Information
- 6.7.4. Future Outlook
- 6.8. PRA Health Sciences
- 6.8.1. Company Overview
- 6.8.2. Financial Information
- 6.8.3. Service Portfolio
- 6.8.4. Future Outlook
- 6.9. Syneos Health
- 6.9.1. Company Overview
- 6.9.2. Financial Information
- 6.9.3. Services Portfolio
- 6.9.4. Future Outlook
- 6.10. Vimta Labs
- 6.10.1. Company Overview
- 6.10.2. Services Portfolio
- 6.10.3. Financial Information
- 6.10.4. Future Outlook
- 6.11. WuXi AppTec
- 6.11.1. Company Overview
- 6.11.2. Financial Information
- 6.11.3. Service Portfolio
- 6.11.4. Future Outlook
7. BENCHMARK ANALYSIS
- 7.1. Chapter Overview
- 7.2. Methodology
- 7.3. Benchmark Analysis: Peer Groups
- 7.3.1. Peer Group I
- 7.3.2. Peer Group II
- 7.3.3. Peer Group III
- 7.3.4. Peer Group IV
- 7.3.5. Peer Group V
- 7.3.6. Peer Group VI
- 7.3.7. Peer Group VII
- 7.3.8. Peer Group VIII
8. PARTNERSHIPS AND COLLABORATIONS
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Biopharmaceutical CROs: Recent Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership Model
- 8.3.2.1. Analysis by Year of Partnership and Type of Partnership Model
- 8.3.3. Most Active Players: Analysis by Number of Partnerships
- 8.3.4. Analysis by Scale of Operation
- 8.3.5. Analysis by Geography
- 8.3.5.1. Country-wise Analysis
- 8.3.5.2. Intercontinental and Intracontinental Agreements
9. MERGERS AND ACQUISITIONS
- 9.1. Chapter Overview
- 9.2. Merger and Acquisition Models
- 9.3. Biopharmaceutical CROs: Mergers and Acquisitions
- 9.3.1. Analysis by Year of Acquisition
- 9.3.2. Analysis by Type of Collaboration
- 9.3.3. Analysis by Geography
- 9.3.3.1. Continent-wise Distribution
- 9.3.3.2. Country-wise Distribution
- 9.3.4. Ownership Change Matrix
- 9.3.5. Most Active Acquirers: Analysis by Number of Acquisitions
- 9.4. Distribution by Key Value Drivers
- 9.4.1. Analysis by Key Value Drivers
- 9.4.2. Analysis by Key Value Drivers and Year of Acquisitions
- 9.5. Valuation Analysis: Acquisition Deal Multiples
10. ATTRACTIVENESS COMPETATIVENESS MATRIX
- 10.1. Chapter Overview
- 10.2. AC Matrix: Overview
- 10.2.1. Strong Business Segment
- 10.2.2. Average Business Segment
- 10.2.3. Weak Business Segment
- 10.3. Analytical Methodology
- 10.4. AC Matrix: Contract Manufacturing Scenario in North America
- 10.5. AC Matrix: Contract Manufacturing Scenario in Europe
- 10.6. AC Matrix: Contract Manufacturing Scenario in Asia Pacific and Middle East
11. MARKET FORECAST
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Global Biopharmaceutical CROs Market
- 11.3.1. Global Biopharmaceutical CROs Market: Distribution by Types of Biologics
- 11.3.2. Global Biopharmaceutical CROs Market: Distribution by Therapeutic Area
- 11.3.3. Global Biopharmaceutical CROs Market: Distribution by Scale of Operation
- 11.3.4. Global Biopharmaceutical CROs Market: Distribution by Geography
- 11.4. Biopharmaceutical CROs Market in North America
- 11.4.1. Biopharmaceutical CROs Market in North America: Distribution by Therapeutic Area
- 11.4.1.1. Biopharmaceutical CROs Market for Oncological Disorders in North America, Till 2035
- 11.4.1.2. Biopharmaceutical CROs Market for Cardiovascular Disorders in North America, Till 2035
- 11.4.1.3. Biopharmaceutical CROs Market for Inflammatory Disorders in North America, Till 2035
- 11.4.1.4. Biopharmaceutical CROs Market for Neurological Disorders in North America, Till 2035
- 11.4.1.5. Biopharmaceutical CROs Market for Other Therapeutic Areas in North America, Till 2035
- 11.4.2. Biopharmaceutical CROs Market in North America: Distribution by Scale of Operation
- 11.4.2.1. Biopharmaceutical CROs Market for Preclinical Operations in North America, Till 2035
- 11.4.2.2. Biopharmaceutical CROs Market for Clinical Operations in North America, Till 2035
- 11.4.1. Biopharmaceutical CROs Market in North America: Distribution by Therapeutic Area
- 11.5. Biopharmaceutical CRO Services Market in Europe
- 11.5.1. Biopharmaceutical CRO Services Market: Distribution by Therapeutic Area in Europe
- 11.5.1.1. Biopharmaceutical CRO Services Market for Oncological Disorders in Europe, Till 2035
- 11.5.1.2. Biopharmaceutical CRO Services Market for Cardiovascular Disorders in Europe, Till 2035
- 11.5.1.3. Biopharmaceutical CRO Services Market for Inflammatory Disorders in Europe, Till 2035
- 11.5.1.4. Biopharmaceutical CRO Services Market for Neurological Disorders in Europe, Till 2035
- 11.5.1.5. Biopharmaceutical CRO Services Market for Other Therapeutic Areas in Europe, Till 2035
- 11.5.2. Biopharmaceutical CRO Services Market: Distribution by Scale of Operation in Europe
- 11.5.2.1. Biopharmaceutical CRO Services Market for Preclinical Operations in Europe, Till 2035
- 11.5.2.2. Biopharmaceutical CRO Services Market for Clinical Operations in Europe, Till 2035
- 11.5.1. Biopharmaceutical CRO Services Market: Distribution by Therapeutic Area in Europe
- 11.6. Biopharmaceutical CRO Services Market in Asia-Pacific
- 11.6.1. Biopharmaceutical CRO Services Market: Distribution by Therapeutic Area in Asia-Pacific
- 11.6.1.1. Biopharmaceutical CRO Services Market for Oncological Disorders in Asia-Pacific, Till 2035
- 11.6.1.2. Biopharmaceutical CRO Services Market for Cardiovascular Disorders in Asia-Pacific, Till 2035
- 11.6.1.3. Biopharmaceutical CRO Services Market for Inflammatory Disorders in Asia-Pacific, Till 2035
- 11.6.1.4. Biopharmaceutical CRO Services Market for Neurological Disorders in Asia-Pacific, Till 2035
- 11.6.1.5. Biopharmaceutical CRO Services Market for Other Therapeutic Areas in Asia-Pacific, Till 2035
- 11.6.2. Biopharmaceutical CRO Services Market: Distribution by Scale of Operation in Asia-Pacific
- 11.6.2.1. Biopharmaceutical CRO Services Market for Preclinical Operations in Asia-Pacific, Till 2035
- 11.6.2.2. Biopharmaceutical CRO Services Market for Clinical Operations in Asia-Pacific, Till 2035
- 11.6.1. Biopharmaceutical CRO Services Market: Distribution by Therapeutic Area in Asia-Pacific
- 11.7. Biopharmaceutical CRO Services Market in Latin America
- 11.8. Biopharmaceutical CRO Services Market in Middle East North America
12. SWOT ANALYSIS
- 12.1. Chapter Overview
- 12.2. Strengths
- 12.3. Weaknesses
- 12.4. Opportunities
- 12.5. Threats
- 12.6. Comparison of SWOT Factors
13. CONCLUDING REMARKS
- 13.1. Chapter Overview

















