
|
시장보고서
상품코드
1762543
특수 CRO 시장 : 업계 동향과 세계 예측 - 대상 치료 영역별, 서비스 유형별, 주요 지역별Specialty CROs Market: Industry Trends and Global Forecasts - Distribution by Target Therapeutic Area, Type of Service and Key Geographical Regions |
||||||
특수 CRO 시장 : 개요
세계의 특수 CRO 시장 규모는 올해 12억 달러에 달했습니다. 이 시장은 예측 기간 중 8.6%의 유리한 CAGR로 성장할 것으로 예측됩니다.
시장 세분화 및 기회 분석은 다음과 같은 매개 변수로 세분화됩니다.
대상 치료 영역
- 종양 질환
- 대사성 질환
- 심혈관 질환
- 당뇨병
- 기타
서비스 유형
- 전임상 서비스
- 임상 서비스
주요 지역
- 북미
- 유럽
- 아시아태평양
- 기타 지역
특수 CRO 시장 : 성장과 동향
수년 동안 바이오 제약 산업은 R&D 아웃소싱에 대한 접근 방식에 큰 변화를 겪어 왔습니다. 전통적인 풀서비스 CRO가 종종 직면하는 한계에 대응하기 위해 전문 CRO(Contract Research Organization)가 유력한 진입자로 부상하고 있습니다. 풀서비스 프로바이더는 광범위한 역량을 제공하지만, 소규모 바이오기업이나 스타트업의 구체적인 니즈에 맞게 서비스를 조정하는 데 어려움을 겪는 경우가 많습니다. 반면, 전문 CRO는 틈새 시장에 초점을 맞추고 종양, 심장병, 대사성 질환, 중추신경계(CNS) 질환과 같은 특정 질환 분야에 집중하면서 표적화된 임상 또는 전임상 서비스 전문성을 제공합니다. 이러한 조직에 일반적으로 위탁하는 서비스에는 약물감시, 표적 평가, 제제 개발, 세포주 개발, 데이터 관리, 프로젝트 관리, 생물통계학 등이 포함됩니다. 또한 특수 CRO는 세계 바이오 제약 부문의 진화하는 니즈에 더 잘 대응하기 위해 서비스 제공의 폭을 넓히고 지역적 범위를 확장하는 전략적 모델을 채택하는 사례가 늘고 있습니다.
특수 CRO 시장 : 주요 인사이트
이 보고서는 세계 특수 CRO 시장의 현황을 조사하고 잠재적인 성장 기회를 파악합니다. 이 보고서의 주요 조사 결과는 다음과 같습니다.
- CRO가 제공하는 다양한 서비스를 통해 CRO는 의약품 개발에 필수적인 플랫폼이 되었습니다.
- 1,000개 이상의 CRO를 대상으로 한 심층 실사 결과, 특정 역량과 서비스 제공 범위에 따라 200개 이상의 특수 CRO가 확인되었습니다.
- 전임상시험을 전문으로 하는 CRO는 전체의 26%이며, 이들 CRO의 서비스 포트폴리오는 고객의 다양한 요구에 부응하고 있습니다.
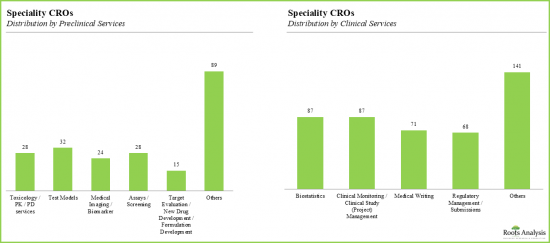
- 독성학 및 약리학은 가장 인기 있는 전임상 서비스이며, CRO의 -14%가 독성학 및 약리학 관련 서비스를 제공합니다.
- 약 32개 기업이 시험 모델 및 의료 영상/바이오마커 기반 분석에 특화되어 있습니다.
- 특수 CRO의 40% 이상이 생물통계 관련 서비스를 제공하고 있으며, 임상 모니터링 및 프로젝트 관리 서비스를 제공하는 특수 CRO가 그 뒤를 잇고 있습니다.
- 특기할 만한 것은 메디컬 라이팅과 약사 관리 및 신청 업무의 아웃소싱이 많다는 점입니다.
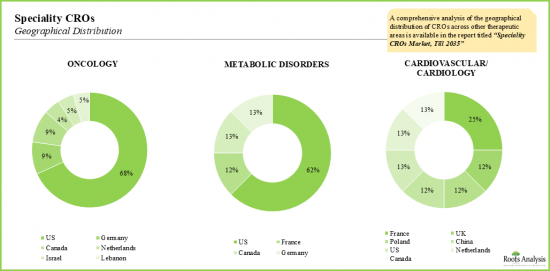
- 전체 시장은 소수의 대형 CRO가 지배하고 있지만, 특수 CRO 부문 시장은 매우 세분화되어 있으며, 미국은 여전히 주요 허브 역할을 하고 있습니다.
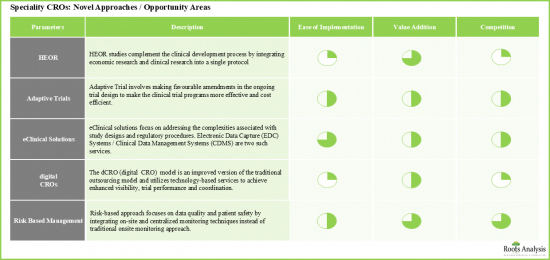
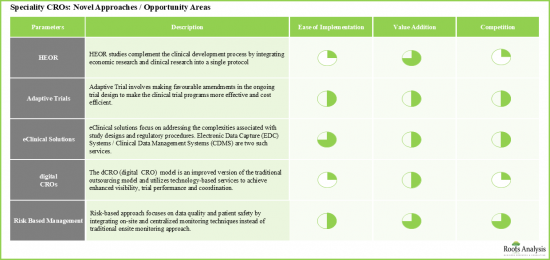
- 새로운 접근 방식과 미개발 기회 영역이 장기적으로 주요 촉진요인으로 부상할 가능성이 높습니다.
- 특히 특수 CRO가 서비스 포트폴리오를 더욱 강화함에 따라 시장은 향후 수년간 성장 모멘텀을 유지할 가능성이 높을 것으로 보입니다.
Specialty CROS 시장의 참여 기업 예
- Accelovance
- Almedis
- Applied Healthcare Resource Management
- Betagenex
- Biospective
- BRI Biopharmaceutical
- BTS Research
- Cardialysis
- CMX Research
- Crown Bioscience
- Dorizoe Lifesciences
- DSP Clinical Research
- DZS Clinical Services
- EthosExcel(TM)
- Fluofarma
- ICRC-Weyer
- Impact Pharmaceutical Services
- IonsGate Preclinical Services
- KIYATEC
- MedSource
- Novella Clinical
- Physiogenex
- Profil Institute
- Redoxis
- RenaSci
- Research Dynamics Consulting
- RxGen
- SDS Clinical
- Spirovation
- Velesco Pharmaceutical Services
목차
제1장 서문
제2장 개요
제3장 서론
- 역사
- 기존 CRO
- 특수 CRO
제4장 특수 CRO : 서론
- 챕터 개요
- 특수 CRO의 중요성
제5장 시장 개요
- 챕터 개요
- 조사 방법
- 특수 CRO : 세계 상황
- 특정 서비스 능력에 중점을 둔 특수 CRO
- 특정의 치료 영역에 중점을 둔 특수 CRO
제6장 특수 CRO : 서비스 중점형
- 챕터 개요
- 전임상 서비스 능력에 중점을 두는 CRO
- BRI Biopharmaceutical Research
- BTS Research
- Dorizoe Lifesciences
- Fluofarma
- KIYATEC
- Redoxis
- Spirovation
- Velesco Pharmaceutical Services
- 임상 서비스 능력에 중점을 두는 CRO
- Almedis
- Applied Healthcare Resource Management
- CMX Research
- DSP Clinical Research
- DZS Clinical Services
- EthosExcel(TM)
- ICRC-Weyer
- Impact Pharmaceutical Services
- Research Dynamics Consulting
- SDS Clinical
제7장 특수 CRO : 치료 영역 중점형
- 챕터 개요
- 종양학에 중점을 둔 특수 CRO
- Accelovance
- Crown Bioscience
- MedSource
- Novella Clinical
- 순환기계/심장병에 중점을 둔 특수 CRO
- Cardialysis
- IonsGate Preclinical Services
- 대사질환에 중점을 둔 특수 CRO
- Betagenex
- Physiogenex
- Profil Institute
- CNS에 중점을 둔 특수 CRO
- Biospective
- RenaSci
- RxGen
제8장 사례 연구 I : 가상 CRO
- 가상 CRO 서론
- Frestedt
- InSymbiosis
- Osiris Pharma
- ProjectPharm
- The Harte Group
- VxP Pharma
제9장 사례 연구 II : 풀서비스 CRO
- 기존 CRO 서론
- Covance
- Medis Research Group
- Quintiles
- Triclinium Clinical Trial Project Management
제10장 시장 예측
- 챕터 개요
- 예측 조사 방법
- 세계의 특수 CRO 시장(-2035년)
- 세계의 특수 CRO 시장(-2035년), 지역별
제11장 향후 기회
제12장 결론
제13장 인터뷰 기록
제14장 부록 1 : 표형식 데이터
제15장 부록 2 : 기업·단체 리스트
제16장 부록 3 : 서비스 맵 용어집
KSA 25.07.10SPECIALTY CROS MARKET: OVERVIEW
As per Roots Analysis, the global specialty CROs market valued at USD 1.2 billion in the current year is anticipated to grow at a lucrative CAGR of 8.6% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Therapeutic Area
- Oncological Disorders
- Metabolic Disorders
- Cardiovascular Disorders
- Diabetes
- Others
Type of Service
- Preclinical Services
- Clinical Services
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
SPECIALTY CROS MARKET: GROWTH AND TRENDS
Over the years, the biopharmaceutical industry has undergone a major transformation in its approach towards R&D outsourcing. Specialty contract research organizations (CROs) have become prominent players, addressing limitations often faced with traditional, full-service CROs. While full-service providers offer a broad range of capabilities, they frequently struggle to tailor their services to the specific needs of small-scale biotech companies and start-ups. In contrast, specialty CROs focus on niche segments, delivering expertise in targeted clinical or preclinical services, while concentrating on a particular disease area such as oncology, heart diseases, metabolic disorders, and central nervous system (CNS) conditions. Services commonly outsourced to these organizations include pharmacovigilance, target evaluation, formulation development, cell line development, data management, project management, biostatistics and others. Further, specialty CROs are also increasingly adopting strategic models to broaden their service offerings and expand geographically, aiming to better serve the evolving needs of the global biopharmaceutical sector.
SPECIALTY CROS MARKET: KEY INSIGHTS
The report delves into the current state of the global specialty CROs market and identifies potential growth opportunities within industry. Some key findings from the report include:
- The wealth of services offered by CROs have made them an indispensable platform for drug development.
- Over 200 speciality CROs were identified after detailed due diligence of more than 1,000 CROs based on the specific capabilities and the range of services they provide.
- With 26% of the organizations specializing in preclinical services, the service portfolio of these CROs caters to a wide array of client requirements.
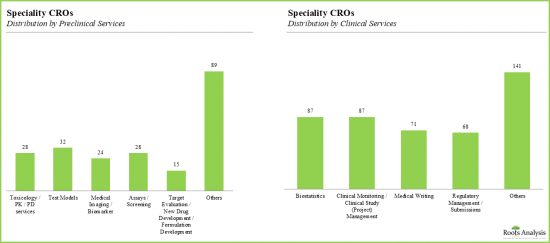
- Toxicology and pharmacology are the most popular preclinical services, with ~14% of the CROs offering the services related to toxicology and pharmacology in their respective portfolios.
- Nearly 32 companies specialize in test models and medical imaging / biomarker-based analysis.
- Over 40% of the speciality CROs offered biostatistics related services, followed by speciality CROs offering clinical monitoring and project management services.
- Notably, medical writing and regulatory management / submission is also frequently outsourced.
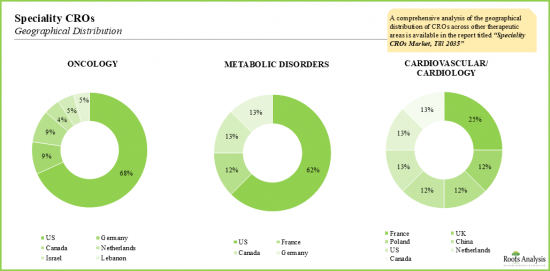
- Though the overall market is dominated by a handful of bigger CROs, the market within the speciality CRO segment is highly fragmented; US continues to remain the primary hub.
- Newer approaches / untapped opportunity areas are likely to emerge as key growth drivers in the long-term.
- Notably, as specialty CROs further establish their service portfolios, it is believed that the market is likely to sustain the growth momentum in the coming years.
Example Players in the Specialty CROS Market
- Accelovance
- Almedis
- Applied Healthcare Resource Management
- Betagenex
- Biospective
- BRI Biopharmaceutical
- BTS Research
- Cardialysis
- CMX Research
- Crown Bioscience
- Dorizoe Lifesciences
- DSP Clinical Research
- DZS Clinical Services
- EthosExcel(TM)
- Fluofarma
- ICRC-Weyer
- Impact Pharmaceutical Services
- IonsGate Preclinical Services
- KIYATEC
- MedSource
- Novella Clinical
- Physiogenex
- Profil Institute
- Redoxis
- RenaSci
- Research Dynamics Consulting
- RxGen
- SDS Clinical
- Spirovation
- Velesco Pharmaceutical Services
SPECIALTY CROS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the specialty CROs market, focusing on key market segments, including [A] target therapeutic area, [B] type of service and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of specialty CROs, based on several relevant parameters, such as [A] geographical location, [B] year of establishment and [C] R&D capabilities.
- Company Profiles: In-depth profiles of specialty CROs focused on specialized preclinical / clinical service offering and particular therapeutic area, based on [A] overview of the company, [B] service portfolio and [C] recent developments and an informed future outlook.
- Case Study 1: A detailed discussion of the virtually integrated, cross-functional outsourcing approach implemented by virtual CROs.
- Case study 2: A detailed discussion of the one-stop-shop approach followed by traditional CROs.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. History
- 3.2. Traditional Contract Research Organizations
- 3.2.1. Services
- 3.2.2. Current Industry Environment
- 3.3. Specialty Contract Research Organizations
4. SPECIALTY CROs: AN INTRODUCTION
- 4.1. Chapter Overview
- 4.2. Importance of Specialty CROs
5. MARKET OVERVIEW
- 5.1. Chapter Overview
- 5.2. Methodology
- 5.3. Specialty CROs: Global Landscape
- 5.3.1. Specialty CROs: The Dramatic Rise
- 5.3.2. Specialty CROs: Distribution by Nature of Specialization
- 5.4. Specialty CROs Focused on Specific Service Capability
- 5.4.1. Landscape is Well Distributed across Preclinical and Clinical Services
- 5.4.2. Specialty CROs: Popular Preclinical Services
- 5.4.3. Specialty CROs: Popular Clinical Services
- 5.5. Specialty CROs Focused on Specific Therapeutic Area(s)
- 5.5.1. Oncology is the Most Researched Therapeutic Area
- 5.5.2. CROs Mostly Located in the US; Europe is a Distant Second
6. SPECIALTY CROs: FOCUSED ON SERVICES
- 6.1. Chapter Overview
- 6.2. CROs Focused on Preclinical Service Capabilities
- 6.2.1. BRI Biopharmaceutical Research
- 6.2.1.1. Company Overview
- 6.2.1.2. Focused Clinical Expertise
- 6.2.1.2.1. API / Clinical Product QC, Dosing Solutions & Materials
- 6.2.1.2.2. Bioanalytical Assays for Clinical Trials
- 6.2.1.2.3. Clinical Trial Equipment Rental & PK Sample Collection Kit
- 6.2.1.2.4. Cynomologus & Rhesus Monkey Hepatocytes & Blood Products
- 6.2.1.2.5. Drug Candidate Early In Vitro & In Vivo Screening
- 6.2.1.2.6. In Vitro DMPK & ADME
- 6.2.1.2.7. In Vivo DMPK & ADME
- 6.2.1.2.8. Patient Tumor-Derived Xenograft Models at Oncograph(TM)
- 6.2.1.2.9. Strategic Development of Botanical Drugs
- 6.2.2. BTS Research
- 6.2.2.1. Company Overview
- 6.2.2.2. Focused Clinical Expertise
- 6.2.2.2.1. Custom Services
- 6.2.2.2.2. Disease Models
- 6.2.2.2.3. In Vitro Services
- 6.2.2.2.4. IND Process
- 6.2.2.2.5. Medical Devices
- 6.2.2.2.6. Pharmacology Services
- 6.2.2.2.7. Toxicology Services
- 6.2.2.3. Additional Information
- 6.2.2.3.1. Recent Developments
- 6.2.2.3.2. Acquisitions
- 6.2.3. Dorizoe Lifesciences
- 6.2.3.1. Company Overview
- 6.2.3.2. Focused Clinical Expertise
- 6.2.3.2.1. Services and Capabilities
- 6.2.3.2.2. Customized Services
- 6.2.4. Fluofarma
- 6.2.4.1. Company Overview
- 6.2.4.2. Focused Clinical Expertise
- 6.2.4.2.1. Assay Development Services
- 6.2.4.2.2. Cell-based screening services
- 6.2.4.2.3. In Vitro Drug Profiling Services and Mechanism of Action (MOA) Studies
- 6.2.4.2.4. Predictive Toxicology Services
- 6.2.4.2.5. In Vivo Studies
- 6.2.4.2.6. Biomarker Analysis Services
- 6.2.4.3. Additional Information
- 6.2.4.3.1. Collaborations
- 6.2.4.3.2. Recent Developments
- 6.2.5. KIYATEC
- 6.2.5.1. Company Overview
- 6.2.5.2. Focused Clinical Expertise
- 6.2.5.2.1. Drug Response Profiling (DRP) Services
- 6.2.5.3. Additional Information
- 6.2.5.3.1. Recent Developments
- 6.2.5.3.2. Funding
- 6.2.6. Redoxis
- 6.2.6.1. Company Overview
- 6.2.6.2. Focused Clinical Expertise
- 6.2.6.2.1. In Vivo Models
- 6.2.6.2.2. In Vitro Services
- 6.2.6.3. Additional Information
- 6.2.6.3.1. Collaborations
- 6.2.6.3.2. Recent Developments
- 6.2.6.3.3. Funding
- 6.2.7. Spirovation
- 6.2.7.1. Company Overview
- 6.2.7.2. Focused Clinical Expertise
- 6.2.7.2.1. Clinical Assessment
- 6.2.7.2.2. Functional Screening and Lead Selection
- 6.2.7.2.3. Target ID and Primary Screening
- 6.2.7.3. Additional Information
- 6.2.7.3.1. Facilities
- 6.2.8. Velesco Pharmaceutical Services
- 6.2.8.1. Company Overview
- 6.2.8.2. Focused Clinical Expertise
- 6.2.8.2.1. Analytical Method Development
- 6.2.8.2.2. cGMP Manufacturing
- 6.2.8.2.3. Drug Formulation Development
- 6.2.8.2.4. Instrumentation
- 6.2.8.2.5. Pharmaceutical Consulting
- 6.2.1. BRI Biopharmaceutical Research
- 6.3. CROs Focused on Clinical Service Capabilities
- 6.3.1. Almedis
- 6.3.1.1. Company Overview
- 6.3.1.2. Focused Clinical Expertise
- 6.3.1.2.1. Biostatistics
- 6.3.1.2.2. Clinical Monitoring
- 6.3.1.2.3. International Clinical Trials
- 6.3.1.2.4. Local Studies
- 6.3.1.2.5. Data Management
- 6.3.1.2.6. Medical Writing
- 6.3.1.2.7. Training Programs
- 6.3.2. Applied Healthcare Resource Management
- 6.3.2.1. Company Overview
- 6.3.2.2. Focused Clinical Services
- 6.3.2.3. Additional Information
- 6.3.2.3.1. Collaborations
- 6.3.3. CMX Research
- 6.3.3.1. Company Overview
- 6.3.3.2. Focused Clinical Expertise
- 6.3.3.2.1. CRO Services
- 6.3.3.2.2. Investigator Network
- 6.3.3.2.3. Investigator-Led Trials (ILTs)
- 6.3.3.2.4. MDapps(TM)
- 6.3.4. DSP Clinical Research
- 6.3.4.1. Company Overview
- 6.3.4.2. Focused Clinical Expertise
- 6.3.4.2.1. Data Management
- 6.3.4.2.2. Monitoring
- 6.3.4.2.3. Project Management
- 6.3.4.2.4. Site Management
- 6.3.4.2.5. Statistics and Medical Writing
- 6.3.4.2.6. Others
- 6.3.4.3. Additional Information
- 6.3.4.3.1. Collaboration
- 6.3.4.3.2. Recent Developments
- 6.3.5. DZS Clinical Services
- 6.3.5.1. Company Overview
- 6.3.5.2. Focused Clinical Services
- 6.3.5.2.1. Biostatistics Support and Consulting
- 6.3.5.2.2. Clinical Data Management
- 6.3.5.2.3. Clinical Monitoring
- 6.3.5.2.4. Clinical Project Management
- 6.3.5.2.5. ClinPlus(R) eClinical Platform
- 6.3.5.2.6. Customized FSP Models
- 6.3.5.2.7. DZS Clinical Sourcing and Staffing
- 6.3.5.2.8. Medical Coding
- 6.3.5.2.9. Medical Writing
- 6.3.5.2.10. Statistical Programming and CDISC Implementation
- 6.3.5.2.11. SOP Authoring
- 6.3.6. EthosExcel(TM)
- 6.3.6.1. Company Overview
- 6.3.6.2. Focused Clinical Expertise
- 6.3.6.2.1. Clinical Trial Services
- 6.3.6.2.2. Clinical Trial Resourcing
- 6.3.6.2.3. Diversity Consulting
- 6.3.6.2.4. Investigator and Trial Site Facilitation Services
- 6.3.6.2.5. Site Management
- 6.3.7. ICRC-Weyer
- 6.3.7.1. Company Overview
- 6.3.7.2. Focused Clinical Expertise
- 6.3.7.2.1. Biostatistics
- 6.3.7.2.2. Clinical Data Management
- 6.3.7.2.3. Medical Review
- 6.3.7.2.4. Medical Writing
- 6.3.7.2.5. Safety Writing and Pharmacovigilance
- 6.3.7.2.6. Scientific Consulting
- 6.3.7.3. Additional Information
- 6.3.7.3.1. Expansion of Service Portfolio
- 6.3.8. Impact Pharmaceutical Services
- 6.3.8.1. Company Overview
- 6.3.8.2. Focused Clinical Expertise
- 6.3.8.2.1. Drug Development Consulting
- 6.3.8.2.2. Early Phase Clinical Trial Management
- 6.3.8.2.3. Medical Writing
- 6.3.8.2.4. Project and Program Management
- 6.3.8.2.5. Regulatory Affairs
- 6.3.8.2.6. Regulatory Operations
- 6.3.9. Research Dynamics Consulting
- 6.3.9.1. Company Overview
- 6.3.9.2. Focused Clinical Expertise
- 6.3.9.2.1. Clinical Monitoring
- 6.3.9.2.2. Consulting
- 6.3.9.2.3. GCP Auditing
- 6.3.9.2.4. Investigator Recruitment
- 6.3.9.2.5. Project Management
- 6.3.9.2.6. Site Management
- 6.3.9.3. Additional Information
- 6.3.9.3.1. Collaborations
- 6.3.10. SDS Clinical
- 6.3.10.1. Company Overview
- 6.3.10.2. Focused Clinical Expertise
- 6.3.10.2.1. Clinical Trial Services
- 6.3.10.2.2. Consulting Services
- 6.3.1. Almedis
7. SPECIALTY CROs: FOCUSED ON THERAPEUTIC AREAS
- 7.1. Chapter Overview
- 7.2. Specialty CROs Focused on Oncology
- 7.2.1. Accelovance
- 7.2.1.1. Company Overview
- 7.2.1.2. Focused Clinical Expertise
- 7.2.1.2.1. CRO Services
- 7.2.1.2.2. Patient Recruitment
- 7.2.1.2.3. Clinical Call Center
- 7.2.1.3. Additional Information
- 7.2.1.3.1. Collaborations
- 7.2.1.3.2. Site Expansion
- 7.2.2. Crown Bioscience
- 7.2.2.1. Company Overview
- 7.2.2.2. Focused Clinical Expertise
- 7.2.2.2.1. Biotherapeutics
- 7.2.2.2.2. Drug Discovery
- 7.2.2.2.3. Metabolic Diseases
- 7.2.2.2.4. Oncology
- 7.2.2.3. Additional Information
- 7.2.2.3.1. Collaborations
- 7.2.2.3.2. Service Expansion
- 7.2.2.3.3. Site Expansion
- 7.2.2.3.4. Recent Developments
- 7.2.2.3.5. Funding
- 7.2.3. MedSource
- 7.2.3.1. Company Overview
- 7.2.3.2. Focused Clinical Expertise
- 7.2.3.2.1. Clinical Data Management and Biostatistics
- 7.2.3.2.2. Clinical Support
- 7.2.3.2.3. Clinical Trial Monitoring
- 7.2.3.2.4. Project Management
- 7.2.3.2.5. Regulatory Affairs Management
- 7.2.3.2.6. Study Start-Up
- 7.2.4. Novella Clinical
- 7.2.4.1. Company Overview
- 7.2.4.2. Focused Clinical Expertise
- 7.2.4.2.1. Clinical Monitoring
- 7.2.4.2.2. Data Management
- 7.2.4.2.3. Data Monitoring Committees
- 7.2.4.2.4. Investigator Strategy & Site Coordination (ISSC)
- 7.2.4.2.5. Medical Monitoring
- 7.2.4.2.6. Medical Writing
- 7.2.4.2.7. Project Management
- 7.2.4.2.8. Quality Assurance
- 7.2.4.2.9. Regulatory Affairs
- 7.2.4.2.10. Safety Management
- 7.2.4.2.11. Steering Committees & Clinical Advisory Boards
- 7.2.4.2.12. Training
- 7.2.4.2.13. Clinical Staffing
- 7.2.4.2.14. Expertise in Oncology across Various Phases of Clinical Trials
- 7.2.1. Accelovance
- 7.3. Specialty CROs Focused on Cardiovascular / Cardiology
- 7.3.1. Cardialysis
- 7.3.1.1. Company Overview
- 7.3.1.2. Focused Clinical Expertise
- 7.3.1.2.1. Trial Services
- 7.3.1.2.2. Core Laboratory
- 7.3.1.2.3. Network of Partners
- 7.3.1.3. Additional Information
- 7.3.2. IonsGate Preclinical Services
- 7.3.2.1. Company Overview
- 7.3.2.2. Focused Clinical Expertise
- 7.3.2.2.1. Cell Based Assays
- 7.3.2.2.2. Isolated Tissue Based Assays
- 7.3.2.2.3. In Vivo Models
- 7.3.1. Cardialysis
- 7.4. Specialty CROs Focused on Metabolic Disorders
- 7.4.1. Betagenex
- 7.4.1.1. Company Overview
- 7.4.1.2. Focused Clinical Expertise
- 7.4.1.2.1. Experimental Services
- 7.4.1.2.2. Consulting Services
- 7.4.1.3. Additional Information
- 7.4.2. Physiogenex
- 7.4.2.1. Company Overview
- 7.4.2.2. Focused Clinical Expertise
- 7.4.2.2.1. Research Services
- 7.4.2.2.2. Consultancy Services
- 7.4.2.3. Additional Information
- 7.4.2.3.1. Collaborations
- 7.4.2.3.2. Recent Developments
- 7.4.3. Profil Institute
- 7.4.3.1. Company Overview
- 7.4.3.2. Focused Clinical Expertise
- 7.4.3.2.1. Clinical Development
- 7.4.3.2.2. Clinical Research
- 7.4.3.2.3. Data Management and Statistical Services
- 7.4.3.2.4. Monitoring, Quality & Compliance
- 7.4.3.2.5. Recruitment Services
- 7.4.3.2.6. Regulatory Affairs
- 7.4.3.2.7. Scientific Services
- 7.4.3.2.8. Experience in Metabolic Disorders
- 7.4.3.3. Additional Information
- 7.4.3.3.1. Collaborations
- 7.4.1. Betagenex
- 7.5. Specialty CROs Focused on CNS
- 7.5.1. Biospective
- 7.5.1.1. Company Overview
- 7.5.1.2. Focused Clinical Expertise
- 7.5.1.2.1. Image Processing Technology
- 7.5.1.2.2. Rodent Models
- 7.5.1.2.3. Animal Imaging
- 7.5.1.2.4. Human Imaging and Clinical Trials
- 7.5.1.2.5. Histology and IHC
- 7.5.2. RenaSci
- 7.5.2.1. Company Overview
- 7.5.2.2. Focused Clinical Expertise
- 7.5.2.2.1. Experimental Services
- 7.5.2.2.2. Consultancy Services
- 7.5.2.3. Additional Information
- 7.5.2.3.1. Recent Developments
- 7.5.3. RxGen
- 7.5.3.1. Company Overview
- 7.5.3.2. Focused Clinical Expertise
- 7.5.3.2.1. Efficacy Models
- 7.5.3.2.2. Custom Model Development
- 7.5.3.2.3. Pharmacokinetics, Pharmacodynamics and Delivery Optimization Services
- 7.5.3.2.4. Toxicology and Safety Pharmacology
- 7.5.3.2.5. Supporting Technologies & Capabilities
- 7.5.3.3. Additional Information
- 7.5.3.3.1. Collaboration
- 7.5.3.3.2. Funding
- 7.5.3.3.3. Recent Developments
- 7.5.1. Biospective
8. CASE STUDY I: VIRTUAL CROs
- 8.1. Introduction to Virtual CROs
- 8.2. Frestedt
- 8.2.1. Company Overview
- 8.2.2. Service Portfolio
- 8.2.2.1. Clinical Research
- 8.2.2.2. Regulatory Affairs
- 8.2.2.3. Quality Systems
- 8.3. InSymbiosis
- 8.3.1. Company Overview
- 8.3.2. Service Portfolio
- 8.3.2.1. Drug Discovery
- 8.3.2.2. Lead Optimization and Efficacy Model
- 8.3.2.3. Non-Clinical Safety Studies and Bioanalysis
- 8.3.2.4. Regulatory IND / IMPD filings
- 8.3.2.5. Document Management
- 8.3.2.6. Phase I / II/ III Clinical Studies
- 8.3.2.7. Collaborations
- 8.3.3. Additional Information
- 8.3.3.1. Recent Developments
- 8.4. Osiris Pharma
- 8.4.1. Company Overview
- 8.4.2. Service Portfolio
- 8.4.2.1. Program Management
- 8.4.2.2. Consultancy
- 8.4.2.3. Non-clinical Assessment and Management
- 8.4.2.4. Preclinical Toxicology and Safety Studies
- 8.4.2.5. Communication and Monitoring
- 8.4.2.6. Writing and Reviewing of Reports
- 8.4.2.7. Other Services
- 8.5. ProjectPharm
- 8.5.1. Company Overview
- 8.5.2. Service Portfolio
- 8.5.2.1. Virtual CRO
- 8.5.2.2. Project Management Consulting / Organizational Project Management
- 8.5.2.3. Vendor Selection
- 8.5.2.4. Financial Audits
- 8.5.2.5. Training
- 8.5.2.6. Rescue Studies
- 8.5.2.7. Study Start-Up
- 8.6. The Harte Group
- 8.6.1. Company Overview
- 8.6.2. Service Portfolio
- 8.6.2.1. Virtual CRO
- 8.6.2.2. Consultation
- 8.6.2.3. Clinical Trial Project Management
- 8.6.2.4. Integrated Project Delivery and Accountability
- 8.7. VxP Pharma
- 8.7.1. Company Overview
- 8.7.2. Service Portfolio
- 8.7.2.1. Chemical Development
- 8.7.2.2. Preclinical
- 8.7.2.3. Preformulation and Solid State Chemistry
- 8.7.2.4. Analytical and Bioanalytical
- 8.7.2.4.1. Analytical Method Development and Validation
- 8.7.2.4.2. Extractables and Leachables Studies
- 8.7.2.4.3. Particle Size Determination
- 8.7.2.4.4. Container-API Compatibility
- 8.7.2.4.5. Material Characterization
- 8.7.2.4.6. Forced Degradation and Stability Studies
- 8.7.2.4.7. Drug Device Compatibility Studies
- 8.7.2.4.8. Microbial Testing
- 8.7.2.4.9. Bioanalytical Testing
- 8.7.2.5. Formulation Development and Clinical Trial materials
- 8.7.2.6. Parenteral and Lyophilized Clinical Trial Materials
- 8.7.2.7. Clinical Packaging and Distribution
- 8.7.2.8. Commercial Services
9. CASE STUDY II: FULL SERVICE CROs
- 9.1. Introduction to Traditional CROs
- 9.2. Covance
- 9.2.1. Company Overview
- 9.2.2. Service Portfolio
- 9.2.2.1. Analytical Services
- 9.2.2.2. Clinical Development
- 9.2.2.3. Clinical Testing
- 9.2.2.4. Consulting
- 9.2.2.5. Health Economics & Market Access
- 9.2.2.6. Lead Optimization
- 9.2.2.7. Manufacturing Services
- 9.2.2.8. Research
- 9.2.2.9. Safety Assessment
- 9.2.3. Collaborations
- 9.3. Medis Research Group
- 9.3.1. Company Overview
- 9.3.2. Focused Clinical Expertise
- 9.3.2.1. Biostatistics
- 9.3.2.2. Data Management
- 9.3.2.3. Medical Writing
- 9.3.2.4. Oncology Expertise
- 9.3.2.5. Pharmacovigilance
- 9.3.2.6. Proofreading and Translation
- 9.3.2.7. RDC System ODM QuaSi(R)
- 9.3.2.8. Regulatory Services
- 9.3.2.9. Scientific Consulting
- 9.3.2.10. Study Documentation
- 9.3.2.11. Study Management
- 9.3.2.12. Study Monitoring
- 9.3.2.13. Clinical Trial Phase Expertise
- 9.3.2.14. Additional Information
- 9.4. Quintiles
- 9.4.1. Company Overview
- 9.4.2. Service Portfolio
- 9.4.2.1. Clinical Trial Execution
- 9.4.2.2. Consulting
- 9.4.2.3. Laboratories
- 9.4.2.4. Real-World and Late Phase
- 9.4.2.5. Patient and Provider Engagement
- 9.4.2.6. Product Marketing and Sales
- 9.4.2.7. Technology Solutions
- 9.4.2.8. Portfolio and Strategic Planning
- 9.4.3. Financial Performance
- 9.4.4. Collaborations
- 9.4.5. Recent Developments
- 9.5. Triclinium Clinical Trial Project Management
- 9.5.1. Company Overview
- 9.5.1.1. Focused Clinical Expertise
- 9.5.1.2. Additional Information
- 9.5.1.2.1. Collaborations
- 9.5.1. Company Overview
10. MARKET FORECAST
- 10.1. Chapter Overview
- 10.2. Forecast Methodology
- 10.3. Global Specialty CROs Market, Till 2035
- 10.4. Regional Specialty CROs Market, Till 2035
11. FUTURE OPPORTUNITIES
- 11.1. Chapter Overview
- 11.2. The Changing Scenario of Outsourcing
- 11.3. Health Economics and Outcomes Research (HEOR) Studies
- 11.4. Adaptive Trial Design
- 11.5. eClinical Solutions
- 11.6. Risk Based Monitoring (RBM)
- 11.7. Digital CRO (dCRO)
12. CONCLUSION
- 12.1. A Widening Portfolio of Services Governed by Industry Constraints
- 12.2. Closer Working Collaboration is the Key to Success
- 12.3. Within Therapeutic Areas, Oncology is the Flagbearer
- 12.4. Due to Several Niche Offerings, Specialty CRO Market Remains Fragmented
- 12.5. The Market of Specialty CROs is Likely to Sustain the Growth Momentum
- 12.6. Untapped Opportunity Areas will Emerge as Key Growth Drivers in the Long-Term
- 12.7. Concluding Remarks
13. INTERVIEW TRANSCRIPTS
- 13.1. Chapter Overview
- 13.2. President, CRO and Outcomes Research, Company A
- 13.3. President, Company B



















