
|
시장보고서
상품코드
1762545
용기 완전성 시험 시장 : 업계 동향과 세계 예측 - 검사 대상 용기 유형별, 검사 대상 용기 재료 유형별, 주요 지역별Container Closure Integrity Testing Market: Industry Trends and Global Forecasts - Distribution by Type of Container Tested, Type of Container Material Tested and Key Geographical Regions |
||||||
용기 완전성 시험 시장 : 개요
세계의 용기 완전성 시험 시장 규모는 2035년까지의 예측 기간 중 7%의 CAGR로 확대하며, 현재 2억 3,600만 달러에서 2035년까지 5억 5,800만 달러로 성장할 것으로 예측됩니다.
시장 규모 및 기회 분석은 다음과 같은 매개 변수로 구분됩니다.
검사 대상 용기 유형
- 바이알
- 주사기
- 카트리지
검사 대상 용기 재질 유형
- 유리
- 플라스틱
주요 지역
- 북미
- 유럽
- 아시아태평양
- 라틴아메리카
- 중동 및 북아프리카
용기 무결성 테스트 시장 : 성장과 동향
용기 무결성 테스트는 잠재적 오염 물질에 대한 무균 장벽을 유지하는 데 중점을 두고 의약품을 포장하는 과정을 말합니다. 환자의 건강과 안전을 고려할 때, 의약품은 미생물 오염이 없어야 하며, 수명주기 동안 안전하게 사용할 수 있어야 합니다. 따라서 제품의 무균성과 안정성을 보장하기 위해 여러 가지 품질 검사가 이루어집니다. 품질 보증이 필요한 단계 중 하나는 의약품의 2차 포장입니다. 포장에 결함이 있으면 의약품이 열화되거나 불안정해져 치료가 제대로 이루어지지 않거나 환자에게 해를 끼칠 수 있는 등 심각한 결과를 초래할 수 있기 때문입니다. 실제로, 용기 폐쇄 시스템의 포장에 약간의 결함이 있어도 의약품의 무균성과 안정성에 악영향을 미치고 반응성 가스, 습도, 미생물의 침입으로 인한 오염을 유발할 수 있습니다. 따라서 의약품의 안전성을 보장하고 위험 가능성을 방지하기 위해 용기 무결성 시험을 실시합니다. 또한 제조부터 사용까지 의약품의 품질이 유지되도록 보장합니다. 실제로 이 기술을 사용하면 미크론 단위의 결함을 감지할 수 있으며, 제품 회수 사례를 최소화할 수 있습니다.
몇 가지 장점에도 불구하고 집중적인 전문 지식의 부족, 공간적 제약, 장비 설치에 대한 막대한 자본 투자는 제조 및 포장 기업에게 몇 가지 도전이 되고 있습니다. 따라서 이 분야의 일부 기업은 서비스 프로바이더에 도움을 요청하고 있습니다. 이는 신규 진출기업 및 기존 이해관계자들에게 서비스를 확장할 수 있는 좋은 기회를 창출하고 있습니다.
용기 무결성 테스트 시장 : 주요 인사이트
이 보고서는 용기 무결성 테스트 시장의 현황을 조사하고 업계의 잠재적인 성장 기회를 파악합니다. 주요 조사 결과는 다음과 같습니다.
- 현재 40개 이상의 서비스 프로바이더가 전 세계에서 용기 무결성 테스트 서비스를 제공합니다.
- 시장 상황의 특징으로, 용기 무결성 시험에는 신규 진출기업과 기존 기업 모두 존재합니다.
- 진출기업은 다양한 지역에 용기 무결성 테스트 시설을 설치하고 있으며, 이러한 시설을 결합하여 분산된 서비스 네트워크를 제공합니다.
- 경쟁 우위를 구축하기 위해, 진출기업은 신뢰할 수 있는 결과를 제공하는 첨단 기술을 통합하여 서비스 포트폴리오를 강화하고 있습니다.
- 용기 무결성 테스트용으로 개발된 장비는 85개 이상이며, 대부분 임상 및 상업용 규모에 대응하고 있습니다.
- 신뢰성 높고 정밀한 시험에 대한 요구가 높아짐에 따라 진화하는 시험 벤치마크에 부합하는 다양한 첨단 기술이 개발되고 있습니다.
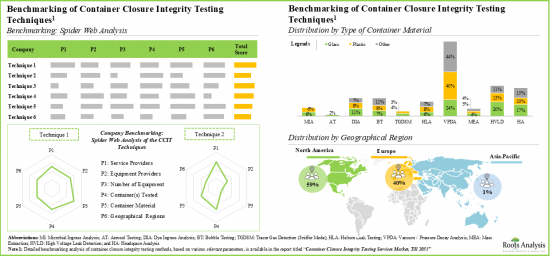
- 장비의 상대적 경쟁력은 동종업계 그룹별로 다릅니다. 이는 제공되는 기능, 사용되는 분석 방법, 테스트되는 용기의 유형 등 여러 가지 매개변수에 따라 달라집니다.
- 용기 마개 무결성 테스트 서비스에 대한 수요는 향후 안정적인 속도로 성장할 것으로 예상되며, 아시아태평양이 전체 수요의 상당 부분을 차지할 것으로 보입니다.
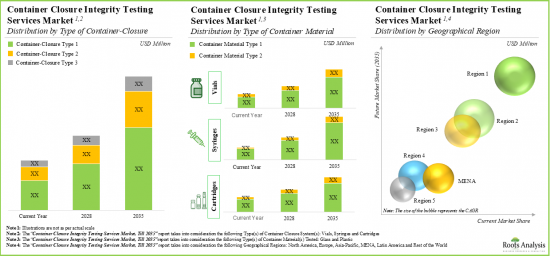
- 시장은 향후 10년간 CAGR 7%를 보일 것으로 예측되며, 그 기회는 다양한 유형의 용기 폐쇄 시스템별, 용기 재료별, 지역별로 잘 분산될 것으로 보입니다.
용기 마개 무결성 검사 시장 : 주요 부문
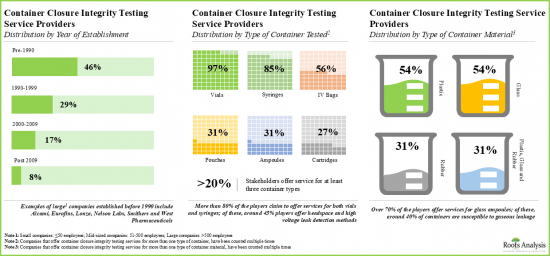
검사 대상 용기 유형에 따라 시장은 바이알, 주사기, 카트리지로 구분됩니다. 현재 바이알이 제약 산업에서 가장 일반적으로 사용되는 용기 유형이기 때문에 바이알 부문이 전 세계 용기 무결성 테스트 시장에서 가장 큰 점유율을 차지하고 있습니다. 이러한 추세는 향후 수년간 변하지 않을 것으로 예측됩니다.
검사 대상 용기 재료 유형에 따라 시장은 유리와 플라스틱으로 구분됩니다. 현재 유리계는 화학적 안정성이 높고 품질 등급이 규제 당국에 의해 인정받고 있으며, 전 세계 용기 무결성 검사 시장에서 가장 높은 비중을 차지하고 있습니다.
주요 지역별로 시장은 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 북아프리카로 구분됩니다. 현재 아시아태평양은 폐쇄 무결성 테스트 시장에서 가장 큰 매출 점유율을 차지하고 있습니다. 이는 이 지역의 제약 산업이 성장하고 의약품 생산이 증가하고 있기 때문입니다. 또한 라틴아메리카 시장은 향후 더 높은 CAGR로 성장할 가능성이 높습니다.
용기 완전성 시험 시장의 참여 기업 예
- Berkshire Sterile Manufacturing
- Confarma
- Curia
- DDL and Nelson Labs
- Eurofins
- SGS
- Stevanato
- Wilco
목차
제1장 서문
제2장 개요
제3장 서론
- 챕터 개요
- 일차 포장 : 용기 폐쇄 시스템
- 용기 폐쇄 시스템의 유형
- 용기 폐쇄 시스템에 관련된 문제
- 용기 완전성 시험(CCI 시험)
- 용기 완전성 시험 방법
- 무균 시험에 대한 용기 완전성 시험의 이점
- CCIT 서비스 프로바이더의 역할
- 향후 전망
제4장 시장 구도
- 챕터 개요
- 용기 완전성 시험 서비스 프로바이더 : 시장 구도
제5장 기업 경쟁력 분석
- 챕터 개요
- 조사 방법
- 주요 파라미터
- 경쟁력 분석 : 북미에서 용기 완전성 시험 서비스를 제공하는 기업
- 경쟁력 분석 : 유럽 및 아시아태평양으로 용기 완전성 시험 서비스를 제공하는 기업
- 경쟁력 분석 : 단일 분석 시설에서 용기 완전성 시험 서비스를 제공하는 기업
- 경쟁력 분석 : 분석 시설 이외에서 용기 완전성 시험 서비스를 제공하는 기업
제6장 북미의 용기 완전성 시험 서비스 프로바이더 : 기업 개요
- 챕터 개요
- Berkshire Sterile Manufacturing
- Curia
- DDL
- Nelson Labs
제7장 유럽의 용기 완전성 시험 서비스 프로바이더 : 기업 개요
- 챕터 개요
- Confarma
- Eurofins
- SGS
- Stevanato
- Wilco
제8장 사례 연구 : 용기 완전성 시험 장비 프로바이더의 시장 구도
- 챕터 개요
- 용기 완전성 시험 장비 : 시장 구도
- 용기 완전성 시험 장비 프로바이더 : 개발자 상황
제9장 제품 경쟁력 분석
- 챕터 개요
- 조사 방법
- 전제/주요 파라미터
- 제품 경쟁력 분석 : 소규모 기업이 제공하는 용기 완전성 시험 장비
- 제품 경쟁력 분석 : 중견 기업이 제공하는 용기 완전성 시험 장비
- 제품 경쟁력 분석 : 대기업이 제공하는 용기 완전성 시험 장비
- 제품 경쟁력 분석 : 북미에 본사를 둔 기업이 제공하는 용기 완전성 시험 장비
- 제품 경쟁력 분석 : 유럽에 본사를 둔 기업이 제공하는 용기 완전성 시험 장비
- 제품 경쟁력 분석 : 아시아태평양에 본사를 둔 기업이 제공하는 용기 완전성 시험 장비
제10장 지역 능력 평가
- 챕터 개요
- 전제와 주요 파라미터
- 북미
- 유럽 및 아시아태평양
- 결론
제11장 벤치마킹 분석
제12장 사례 연구 : 의약품 포장용 로봇 공학
- 챕터 개요
- 제약 업계에서 로봇의 역할
- 제약 업계용 로봇을 제공하는 기업
- 의약품 포장용 로봇 시스템을 통합한 장비를 제공하는 기업
제13장 수요 분석
- 챕터 개요
- 범위와 조사 방법
- 2035년까지 용기 완전성 시험 서비스의 세계 수요
- 결론
제14장 시장 예측과 기회 분석
- 챕터 개요
- 예측 조사 방법과 주요 전제조건
- 세계의 용기 폐쇄부 완전성 시험 서비스 시장(-2035년)
제15장 SWOT 분석
제16장 결론
제17장 이그제큐티브 인사이트
제18장 부록 1 : 표형식 데이터
제19장 부록 2 : 기업·단체 리스트
KSA 25.07.10CONTAINER CLOSURE INTEGRITY TESTING MARKET: OVERVIEW
As per Roots Analysis, the global container closure integrity testing market is estimated to grow from USD 236 million in the current year to USD 558 million by 2035, at a CAGR of 7% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Container Tested
- Vials
- Syringes
- Cartridges
Type of Container Material Tested
- Glass
- Plastic
Key Geographical Regions
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and North Africa
CONTAINER CLOSURE INTEGRITY TESTING MARKET: GROWTH AND TRENDS
Container closure integrity testing refers to the process of packaging a drug product while focusing on maintaining the sterile barrier against potential contaminants. Considering patients' health and safety, the drug products are expected to be free from microbial contamination and safe for use throughout its life cycle. Therefore, several quality checks are performed in order to ensure the product's sterility and stability. One of the stages that require quality assurance is secondary packaging of the drug product, as compromised packaging could have serious consequences, such as drugs may degrade or become unstable, leading to ineffective treatments and potential harm to patients. In fact, even minor defects in the packaging of container closure system could adversely affect the sterility and stability of the drug, allowing contamination via reactive gases, humidity and microbial ingress. Thus, to ensure the safety of drug and prevent any chance of risk, container closure integrity testing is performed. It also ensures that the quality of a drug product is maintained from manufacturing to its use. In fact, by using this technique, detection of micron defects is possible which facilitates minimum cases of product recalls.
Despite several advantages, lack of focused expertise, spatial limitation and huge capital investment in the installation of equipment poses a few challenges for the manufacturing and packaging companies. Therefore, several companies in this area are seeking assistance from service providers. This has created lucrative opportunities for upcoming and existing stakeholders to expand their offerings.
CONTAINER CLOSURE INTEGRITY TESTING MARKET: KEY INSIGHTS
The report delves into the current state of the container closure integrity testing market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, over 40 service providers provide container closure integrity testing services across the globe; majority of the service providers use deterministic methods for testing purposes.
- The market landscape features the presence of both new entrants and well-established players for testing integrity of container closure systems.
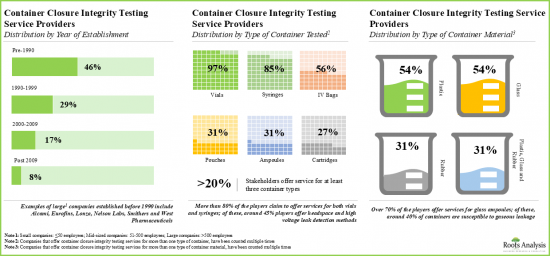
- Players have established their container closure integrity testing facilities in various geographical locations; these facilities, combined, offer a well distributed services network.
- In pursuit of building a competitive edge, players are enhancing their service portfolio by integrating advanced technologies that offer reliable results.
- Over 85 pieces of equipment have been developed for container closure integrity testing; majority of the equipment is designed for both clinical / commercial scale operations.
- Given the increasing demand for reliable and precise testing, various advanced techniques have been developed to comply to the evolving testing benchmarks.
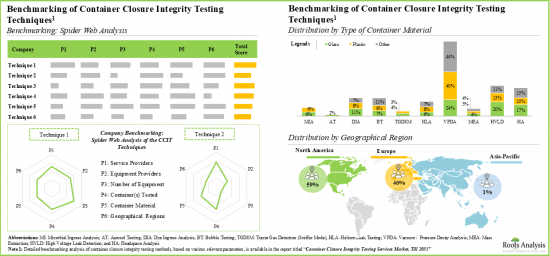
- The relative competitiveness of equipment varies across different peer groups; this is driven by several parameters, such as features offered, the analytical methods used, and type of container tested.
- The demand for container closure integrity testing services is expected to grow at a steady pace in the foreseen future; Asia-Pacific will drive a significant proportion of the overall demand.
- The market is expected to grow at a CAGR of 7% in the coming decade; the opportunity is likely to be well distributed across various types of container closure systems, container materials and geographical regions.
CONTAINER CLOSURE INTEGRITY TESTING MARKET: KEY SEGMENTS
Vials Occupy the Largest Share of the Global Container Closure Integrity Testing Market
Based on the type of container tested, the market is segmented into vials, syringes and cartridges. At present, the vials segment holds the maximum share of the global container closure integrity testing market owing to the fact that vials are the most commonly used type of container in the pharmaceutical industry. This trend is likely to remain the same in the coming years.
By Type of Container Material Tested, Glass System Accounts for the largest share of the Global Container Closure Integrity Testing Market
Based on the type of container material tested, the market is segmented into glass and plastic materials. Currently, the glass segment captures the highest proportion of the global container closure integrity testing market due to its higher chemical stability and regulatory acceptance in terms of its quality grade.
Asia-Pacific Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Latin America, and Middle East and North Africa. Currently, Asia-Pacific dominates the closure integrity testing market and accounts for the largest revenue share. This can be attributed to the growing pharmaceutical industry and increasing production of pharmaceutical products in this region. Further, the market in Latin America is likely to grow at a higher CAGR in the coming future.
Example Players in the Container Closure Integrity Testing Market
- Berkshire Sterile Manufacturing
- Confarma
- Curia
- DDL and Nelson Labs
- Eurofins
- SGS
- Stevanato
- Wilco
CONTAINER CLOSURE INTEGRITY TESTING MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global container closure integrity testing market, focusing on key market segments, including [A] type of container tested, [B] type of container material tested and [C] key geographical regions.
- Market Landscape: A comprehensive evaluation of container closure integrity testing service providers, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] location of analytical facilities, [E] type(s) of analytical method(s) offered, [F] type(s) of probabilistic method(s), [F] type(s) of deterministic method(s) offered, [G] leakage susceptibility, [H] type(s) of container(s) tested and [I] accreditations.
- Company Competitiveness Analysis: A comprehensive competitive analysis of container closure integrity testing service providers, examining factors, such as [A] supplier power and [B] service strength.
- Company Profiles: In-depth profiles of key players providing container closure integrity testing, focusing on [A] overview of the company, [B] financial information (if available), [C] service portfolio, [D] location of analytical facilities, [E] type(s) of analytical method(s) used, [F] types(s) of container(s) tested and [G] recent developments and an informed future outlook.
- Case Study: A detailed assessment of equipment used by various manufacturers to test container closure integrity, focusing on key features, such as [A] type(s) of analytical method(s) offered, [B] type(s) of container(s) tested and [C] container material(s) of container closure integrity testing technologies.
- Product Competitiveness Analysis: A comprehensive competitive analysis of container closure integrity testing equipment, examining factors, such as [A] product strength and [B] product applicability.
- Regional Capability Assessment: A detailed assessment of container closure integrity testing capability across key geographies, based on a number of parameters, such as [A] number of container closure integrity testing service providers, [B] number of analytical testing facilities, [C] number of container closure integrity technology manufacturers, [D] number of container closure integrity testing technologies, [E] number of patents and [F] demand of container closure integrity testing service.
- Benchmarking Analysis: An insightful analysis of the various container closure integrity testing analytical techniques, based on various parameters, such as [A] the number of service providers offering analytical technique for testing purpose, [B] equipment providers developing equipment for particular technique, [C] number of equipment, [D] number of container closure systems tested and [E] benchmarking of analytical techniques.
- Demand Analysis: An in-depth analysis of the current and future demand of container closure integrity testing service, based on various relevant parameters, such as [A] type of container closure system tested and [B] type of material used, across different regions
- SWOT Analysis: An analysis of industry affiliated trends, opportunities and challenges, which are likely to impact the evolution of container closure integrity testing market; it includes a Harvey ball analysis, assessing the relative impact of each SWOT parameter on industry dynamics.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Primary Packaging: Container Closure Systems
- 3.3. Types of Container Closure Systems
- 3.4. Problems Related to Container Closure Systems
- 3.4.1. Types of Contamination
- 3.4.2. Defects in Container Closure Systems
- 3.5. Container Closure Integrity testing (CCI Testing)
- 3.6. Methods of Container Closure Integrity Testing
- 3.7. Advantages of Container Closure Integrity Testing Over Sterility Testing
- 3.8. Role of CCIT Service Providers
- 3.9. Future Perspective
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Container Closure Integrity Testing Service Providers: Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Company Size and Location of Headquarters
- 4.2.5. Analysis by Location of Analytical Facilities
- 4.2.6. Analysis by Type(s) of Analytical Method(s) Offered
- 4.2.6.1. Analysis by Type(s) of Probabilistic Method(s) Offered
- 4.2.6.2. Analysis by Type(s) of Deterministic Method(s) Offered
- 4.2.7. Analysis by Leakage Susceptibility
- 4.2.8. Analysis by Type(s) of Container(s) Tested
- 4.2.9. Analysis by Type(s) of Container Material(s) Tested
- 4.2.10. Analysis by Accreditation(s) Received
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Methodology
- 5.3. Key Parameters
- 5.4. Competitiveness Analysis: Companies Offering Container Closure Integrity Testing Services in North America
- 5.5. Competitiveness Analysis: Companies Offering Container Closure Integrity Testing Services in Europe and Asia-Pacific
- 5.6. Competitiveness Analysis: Companies Offering Container Closure Integrity Testing Services in One Analytical Facility
- 5.7. Competitiveness Analysis: Companies Offering Container Closure Integrity Testing Services in More than Analytical Facility
6. CONTAINER CLOSURE INTEGRITY TESTING SERVICE PROVIDERS IN NORTH AMERICA: COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Berkshire Sterile Manufacturing
- 6.2.1. Company Overview
- 6.2.2. Financial Information
- 6.2.3. Service Portfolio
- 6.2.4. Recent Developments and Future Outlook
- 6.3. Curia
- 6.3.1. Company Overview
- 6.3.2. Financial Information
- 6.3.3. Service Portfolio
- 6.3.4. Recent Developments and Future Outlook
- 6.4. DDL
- 6.4.1. Company Overview
- 6.4.2. Financial Information
- 6.4.3. Service Portfolio
- 6.4.4. Recent Developments and Future Outlook
- 6.5. Nelson Labs
- 6.5.1. Company Overview
- 6.5.2. Financial Information
- 6.5.3. Service Portfolio
- 6.5.4. Recent Developments and Future Outlook
7. CONTAINER CLOSURE INTEGRITY TESTING SERVICE PROVIDERS IN EUROPE: COMPANY PROFILES
- 7.1. Chapter Overview
- 7.2. Confarma
- 7.2.1. Company Overview
- 7.2.2. Financial Information
- 7.2.3. Service Portfolio
- 7.2.4. Recent Developments and Future Outlook
- 7.3. Eurofins
- 7.3.1. Company Overview
- 7.3.2. Financial Information
- 7.3.3. Service Portfolio
- 7.3.4. Recent Developments and Future Outlook
- 7.4. SGS
- 7.4.1. Company Overview
- 7.4.2. Financial Information
- 7.4.3. Service Portfolio
- 7.4.4. Recent Developments and Future Outlook
- 7.5. Stevanato
- 7.5.1. Company Overview
- 7.5.2. Financial Information
- 7.5.3. Service Portfolio
- 7.5.4. Recent Developments and Future Outlook
- 7.6. Wilco
- 7.6.1. Company Overview
- 7.6.2. Financial Information
- 7.6.3. Service Portfolio
- 7.6.4. Recent Developments and Future Outlook
8. CASE STUDY: MARKET LANDSCAPE OF CONTAINER CLOSURE INTEGRITY TESTING EQUIPMENT PROVIDERS
- 8.1. Chapter Overview
- 8.2. Container Closure Integrity Testing Equipment: Market Landscape
- 8.2.1. Analysis by Scale of Operation
- 8.2.2. Analysis by Key Features
- 8.2.3. Analysis by Type(s) of Analytical Method(s) Offered
- 8.2.4. Analysis by Type(s) of Container Material(s) Tested
- 8.2.5. Analysis by Type(s) of Container(s) Tested
- 8.3. Container Closure Integrity Testing Equipment Providers: Developer Landscape
- 8.3.1. Analysis by Year of Establishment
- 8.3.2. Analysis by Company Size
- 8.3.3. Analysis by Location of Headquarters
- 8.3.4. Analysis by Company Size and Location of Headquarters
- 8.3.5. Leading Developers: Analysis by Number of Products
9. PRODUCT COMPETITIVENESS ANALYSIS
- 9.1. Chapter Overview
- 9.2. Methodology
- 9.3. Assumptions / Key Parameters
- 9.4. Product Competitiveness Analysis: Container Closure Integrity Testing Equipment Offered by Small Players
- 9.5. Product Competitiveness Analysis: Container Closure Integrity Testing Equipment Offered by Mid-Sized Players
- 9.6. Product Competitiveness Analysis: Container Closure Integrity Testing Equipment Offered by Large Players
- 9.7. Product Competitiveness Analysis: Container Closure Integrity Testing Equipment Offered by Players Headquartered in North America
- 9.8. Product Competitiveness Analysis: Container Closure Integrity Testing Equipment Offered by Players Headquartered in Europe
- 9.9. Product Competitiveness Analysis: Container Closure Integrity Testing Equipment Offered by Players Headquartered in Asia-Pacific
10. REGIONAL CAPABILITY ASSESSMENT
- 10.1. Chapter Overview
- 10.2. Assumptions and Key Parameters
- 10.3. Container Closure Integrity Testing Capabilities in North America
- 10.4. Container Closure Integrity Testing Capabilities in Europe and Asia-Pacific
- 10.5. Concluding Remarks
11. BENCHMARKING ANALYSIS
- 11.1. Chapter Overview
- 11.2. Methodology Assumptions and Key Assumption
- 11.3. Advantages and Disadvantages of Deterministic Methods
- 11.4. Advantages and Disadvantages of Probabilistic Methods
- 11.5. Benchmarking of CCIT Techniques
- 11.5.1. Distribution by Type(s) of Container(s) Tested
- 11.5.2. Distribution by Type of Container Material Tested
- 11.5.3. Distribution by Key Geographical Regions
12. CASE STUDY: ROBOTICS IN PHARMACEUTICAL PACKAGING
- 12.1. Chapter Overview
- 12.2. Role of Robots in the Pharmaceutical Industry
- 12.2.1. Key Considerations for Selecting a Robotic System
- 12.2.2. Advantages of Robotic Systems
- 12.2.3. Disadvantages of Robotic Systems
- 12.3. Companies Providing Robots for Pharmaceutical Industry
- 12.4. Companies Providing Equipment Integrated with Robotic Systems for Pharmaceutical Packaging
- 12.4.1. Aseptic Technologies
- 12.4.1.1. Crystal(R) L1 Robot Line
- 12.4.1.2. Crystal(R) SL1 Robot Line
- 12.4.2. AST
- 12.4.2.1. ASEPTiCell(R) Series
- 12.4.2.2. ASEPTiCell(R) VSM-25
- 12.4.3. Bosch Packaging Technology
- 12.4.3.1. ATO
- 12.4.4. Dara Pharmaceutical Packaging
- 12.4.4.1. SYX-E CARTRIDGE + RABS
- 12.4.5. Fedegari Group
- 12.4.5.1. Fedegari Isolator
- 12.4.6. IMA
- 12.4.6.1. INJECTA
- 12.4.6.2. STERI LIF3
- 12.4.7. Steriline
- 12.4.7.1. Nest Filling Line RNFM
- 12.4.8. Vanrx Pharmasystems
- 12.4.8.1. Microcell Vial Filler
- 12.4.8.2. SA25 Aseptic Filling Workcell
- 12.4.1. Aseptic Technologies
13. DEMAND ANALYSIS
- 13.1. Chapter Overview
- 13.2. Scope and Methodology
- 13.3. Global Demand for Container Closure Integrity Testing Services, Till 2035
- 13.3.1. Analysis by Type of Container
- 13.3.1.1. Global Demand for Vials, Till 2035
- 13.3.1.2. Global Demand for Syringes, Till 2035
- 13.3.1.3. Global Demand for Cartridges, Till 2035
- 13.3.2. Analysis by Type of Container Material
- 13.3.2.1. Global Demand for Glass Containers, Till 2035
- 13.3.2.1.1. Global Demand for Glass Vials, Till 2035
- 13.3.2.1.2. Global Demand for Glass Syringes, Till 2035
- 13.3.2.1.3. Global Demand for Glass Cartridges, Till 2035
- 13.3.2.2. Global Demand for Plastic Containers, Till 2035
- 13.3.2.2.1. Global Demand for Plastic Vials, Till 2035
- 13.3.2.2.2. Global Demand for Plastic Syringes, Till 2035
- 13.3.2.2.3. Global Demand for Plastic Cartridges, Till 2035
- 13.3.2.1. Global Demand for Glass Containers, Till 2035
- 13.3.3. Analysis by Geography
- 13.3.3.1. Demand for Container Closure Integrity Testing Services in North America, Till 2035
- 13.3.3.1.1. Demand for Vials in North America, Till 2035
- 13.3.3.1.2. Demand for Syringes in North America, Till 2035
- 13.3.3.1.3. Demand for Cartridges in North America, Till 2035
- 13.3.3.2. Demand for Container Closure Integrity Testing Services in Europe, Till 2035
- 13.3.3.2.1. Demand for Vials in Europe, Till 2035
- 13.3.2.2.2. Demand for Syringes in Europe, Till 2035
- 13.3.3.2.3. Demand for Cartridges in Europe, Till 2035
- 13.3.3.3. Demand for Container Closure Integrity Testing Services in Asia Pacific, Till 2035
- 13.3.3.3.1. Demand for Vials in Asia Pacific, Till 2035
- 13.3.3.3.2. Demand for Syringes in Asia Pacific, Till 2035
- 13.3.3.3.3. Demand for Cartridges in Asia Pacific, Till 2035
- 13.3.3.4. Demand for Container Closure Integrity Testing Services in Middle East and North Africa, Till 2035
- 13.3.3.4.1. Demand for Vials in Middle East and North Africa, Till 2035
- 13.3.3.4.2. Demand for Syringes in Middle East and North Africa, Till 2035
- 13.3.3.4.3. Demand for Cartridges in Middle East and North Africa, Till 2035
- 13.3.3.5. Demand for Container Closure Integrity Testing Services in Latin America, Till 2035
- 13.3.3.5.1. Demand for Vials in Latin America, Till 2035
- 13.3.3.5.2. Demand for Syringes in Latin America, Till 2035
- 13.3.3.5.3. Demand for Cartridges in Latin America, Till 2035
- 13.3.3.6. Demand for Container Closure Integrity Testing Services in Rest of the World, Till 2035
- 13.3.3.6.1. Demand for Vials in Rest of the World, Till 2035
- 13.3.3.6.2. Demand for Syringes in Rest of the World, Till 2035
- 13.3.3.6.3. Demand for Cartridges in Rest of the World, Till 2035
- 13.3.3.1. Demand for Container Closure Integrity Testing Services in North America, Till 2035
- 13.3.1. Analysis by Type of Container
- 13.4. Concluding Remarks
14. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 14.1. Chapter Overview
- 14.2. Forecast Methodology and Key Assumptions
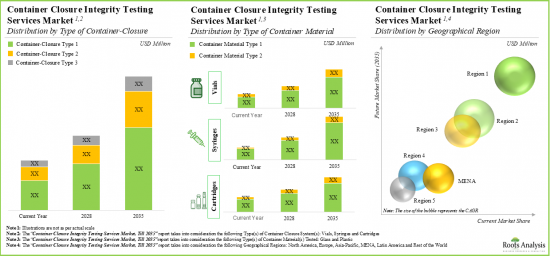
- 14.3. Global Container Closure Integrity Testing Services Market, Till 2035
- 14.3.1. Container Closure Integrity Testing Services Market, Till 2035: Distribution by Type of Container
- 14.3.1.1. Container Closure Integrity Testing Services Market for Vials, Till 2035
- 14.3.1.2. Container Closure Integrity Testing Services Market for Syringes, Till 2035
- 14.3.1.3. Container Closure Integrity Testing Services Market for Cartridges, Till 2035
- 14.3.2. Container Closure Integrity Testing Services Market, Till 2035: Distribution by Type of Container Material Tested
- 14.3.2.1. Container Closure Integrity Testing Services Market for Glass Containers, Till 2035
- 14.3.2.1.1. Container Closure Integrity Testing Services Market for Glass Vials, Till 2035
- 14.3.2.1.2. Container Closure Integrity Testing Services Market for Glass Syringes, Till 2035
- 14.3.2.1.3. Container Closure Integrity Testing Services Market for Glass Cartridges, Till 2035
- 14.3.2.2. Container Closure Integrity Testing Services Market for Plastic Containers Market, Till 2035
- 14.3.2.2.1. Container Closure Integrity Testing Services Market for Plastic Vials, Till 2035
- 14.3.2.2.2. Container Closure Integrity Testing Services Market for Plastic Syringes, Till 2035
- 14.3.2.2.3. Container Closure Integrity Testing Services Market for Plastic Cartridges, Till 2035
- 14.3.2.1. Container Closure Integrity Testing Services Market for Glass Containers, Till 2035
- 14.3.3. Container Closure Integrity Testing Services Market, Till 2035: Distribution by Geography
- 14.3.3.1. Container Closure Integrity Testing Services Market in North America, Till 2035
- 14.3.3.1.1. Container Closure Integrity Testing Services Market for Vials in North America, Till 2035
- 14.3.3.1.2. Container Closure Integrity Testing Services Market for Syringes in North America, Till 2035
- 14.3.3.1.3. Container Closure Integrity Testing Services Market for Cartridges in North America, Till 2035
- 14.3.3.1. Container Closure Integrity Testing Services Market in Europe, Till 2035
- 14.3.3.1.1. Container Closure Integrity Testing Services Market for Vials in Europe, Till 2035
- 14.3.3.1.2. Container Closure Integrity Testing Services Market for Syringes in Europe, Till 2035
- 14.3.3.1.3. Container Closure Integrity Testing Services Market for Cartridges in Europe, Till 2035
- 14.3.3.1. Container Closure Integrity Testing Services Market in Asia-Pacific, Till 2035
- 14.3.3.1.1. Container Closure Integrity Testing Services Market for Vials in Asia-Pacific, Till 2035
- 14.3.3.1.2. Container Closure Integrity Testing Services Market for Syringes in Asia-Pacific, Till 2035
- 14.3.3.1.3. Container Closure Integrity Testing Services Market for Cartridges in Asia-Pacific, Till 2035
- 14.3.3.1. Container Closure Integrity Testing Services Market in Middle East and North Africa, Till 2035
- 14.3.3.1.1. Container Closure Integrity Testing Services Market for Vials in Middle East and North Africa, Till 2035
- 14.3.3.1.2. Container Closure Integrity Testing Services Market for Syringes in Middle East and North Africa, Till 2035
- 14.3.3.1.3. Container Closure Integrity Testing Services Market for Cartridges in Middle East and North Africa, Till 2035
- 14.3.3.1. Container Closure Integrity Testing Services Market in Latin America, Till 2035
- 14.3.3.1.1. Container Closure Integrity Testing Services Market for Vials in Latin America, Till 2035
- 14.3.3.1.2. Container Closure Integrity Testing Services Market for Syringes in Latin America, Till 2035
- 14.3.3.1.3. Container Closure Integrity Testing Services Market for Cartridges in Latin America, Till 2035
- 14.3.3.1. Container Closure Integrity Testing Services Market in Rest of the World, Till 2035
- 14.3.3.1.1. Container Closure Integrity Testing Services Market for Vials in Rest of the World, Till 2035
- 14.3.3.1.2. Container Closure Integrity Testing Services Market for Syringes in Rest of the World, Till 2035
- 14.3.3.1.3. Container Closure Integrity Testing Services Market for Cartridges in Rest of the World, Till 2035
- 14.3.3.1. Container Closure Integrity Testing Services Market in North America, Till 2035
- 14.3.1. Container Closure Integrity Testing Services Market, Till 2035: Distribution by Type of Container
15. SWOT ANALYSIS
- 15.1. Chapter Overview
- 15.2. Strengths
- 15.3. Weaknesses
- 15.4. Opportunities
- 15.5. Threats
- 15.6. Comparison of SWOT Factors
16. CONCLUSION
- 16.1. Chapter Overview



















